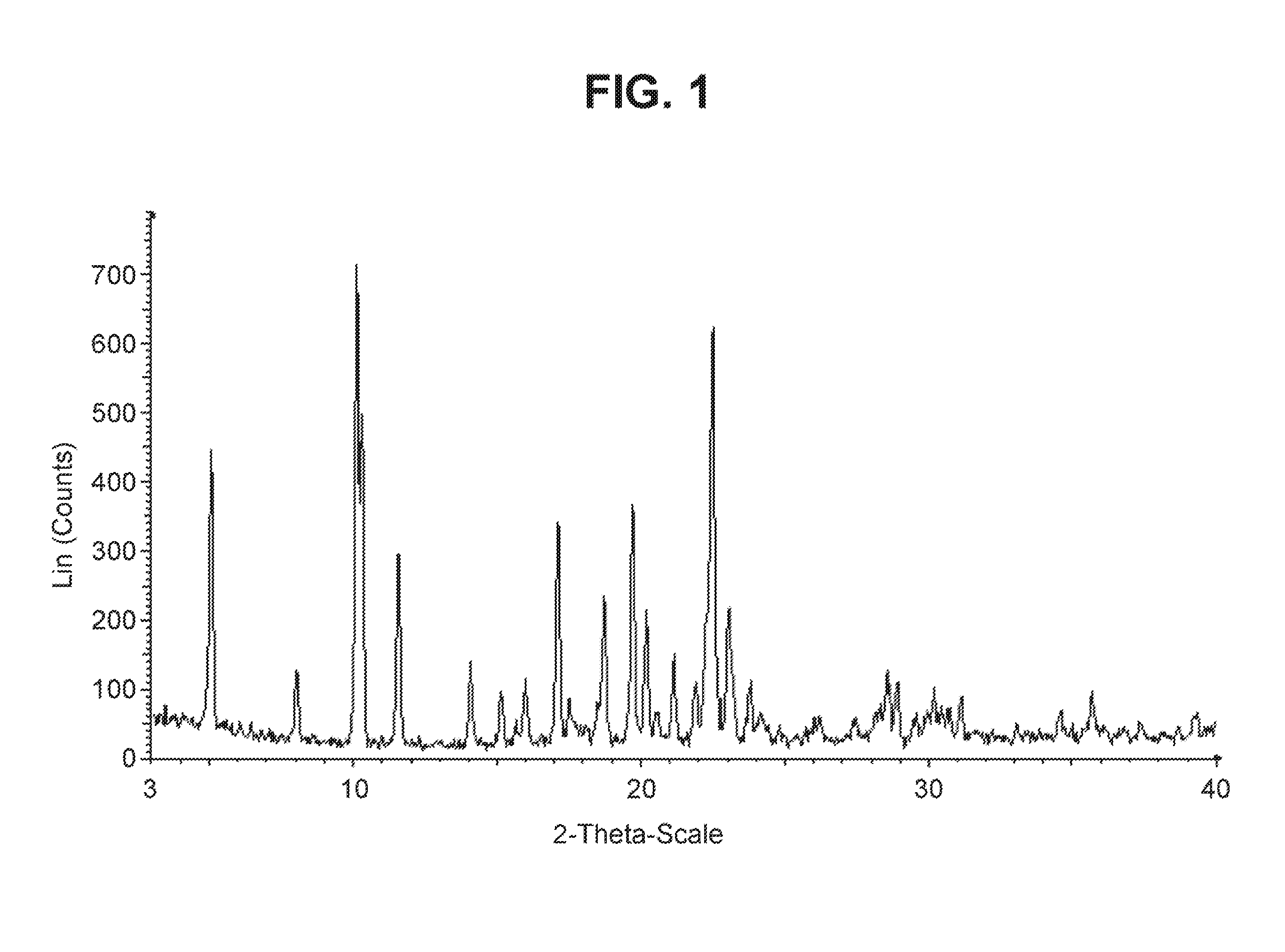
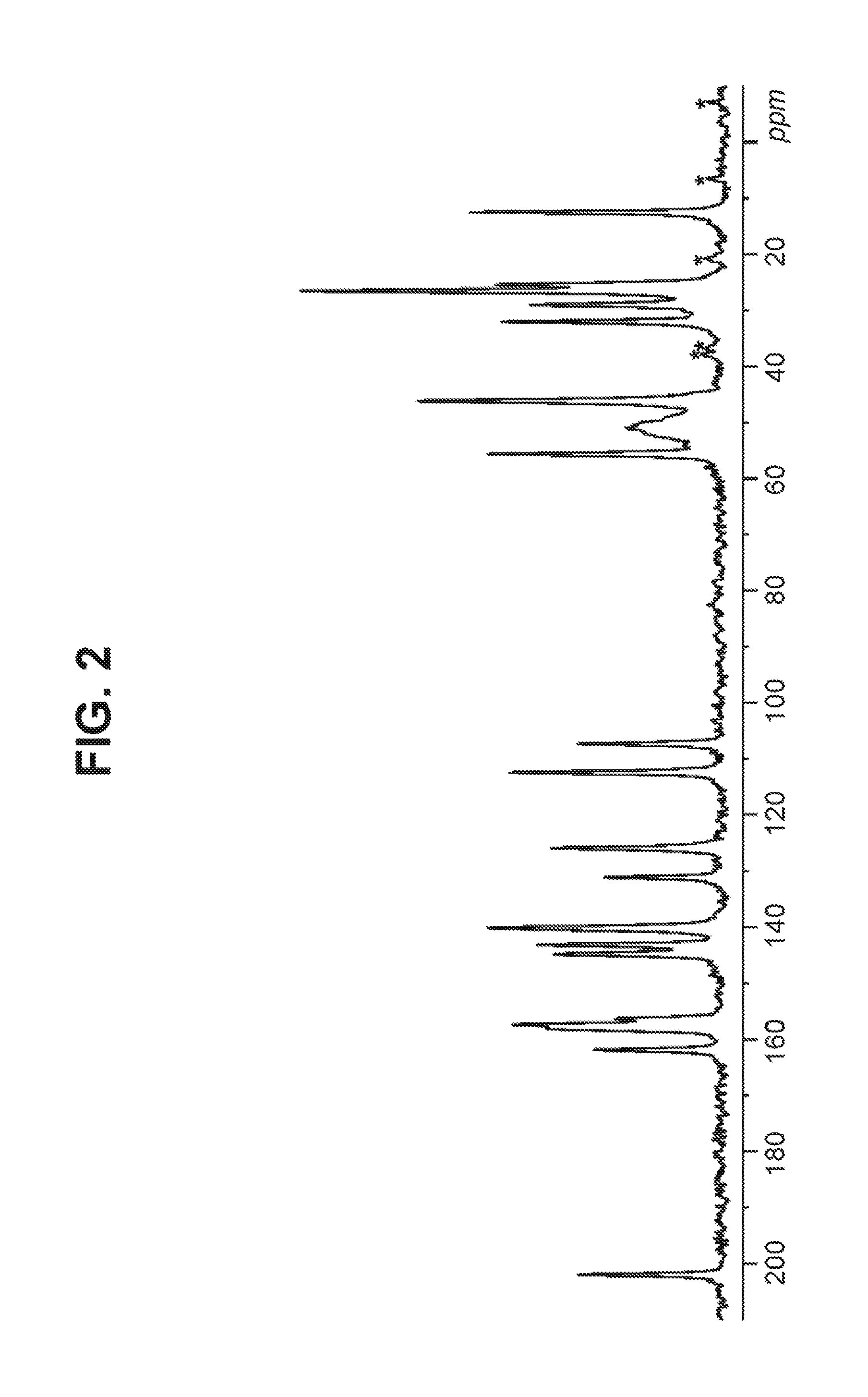
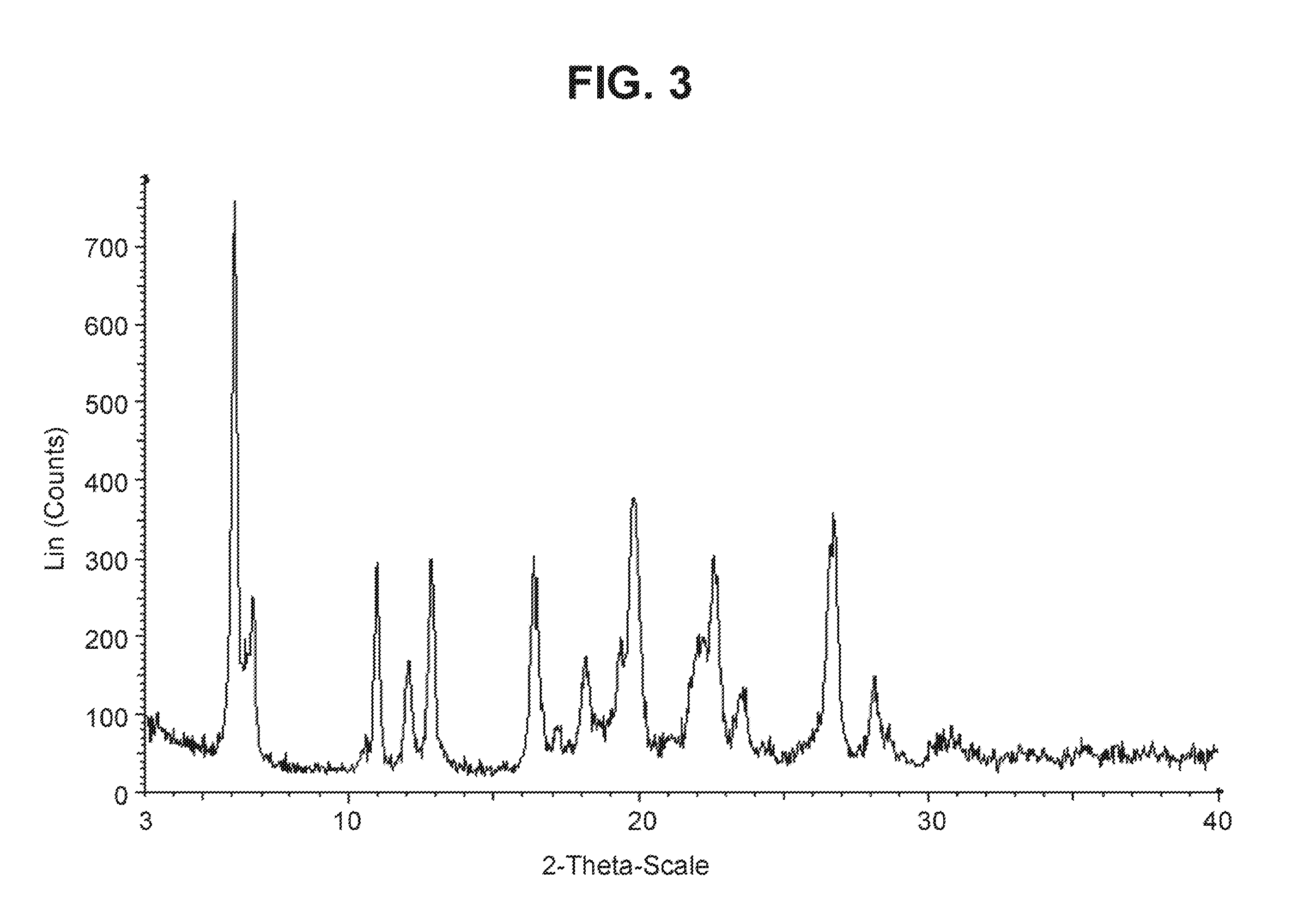
Solid forms of a selective cdk4/6 inhibitor
a selective cdk4/6, solid form technology, applied in the direction of cellulosic plastic layered products, natural mineral layered products, drug compositions, etc., can solve the problems of difficult dispersion of hard agglomerates, unsuitable for further development, and the use of free bases as a base presented challenges for pharmaceutical development, so as to improve yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 4-(6-amino-pyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester
[0161]
Step A. Preparation of 4-(6-nitro-pyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester
[0162]To a vessel was added 5-bromo-2-nitropyridine (10.0 g, 1.0 equiv.) along with DMSO (25 mL, 2.5 vol). N-Boc piperazine (13.8 g, 1.5 equiv.) was added, followed by triethylamine (7.5 g, 1.5 equiv.) and LiCl (2.1 g, 1.0 equiv.). The mixture was warmed to 60-65° C. for a minimum of 12 hours.
[0163]Water (5 mL, 0.5 vol) was added slowly to the vessel at 60-65° C. The mixture was kept at 60-65° C. for one hour, then cooled to room temperature. The slurry was kept at 20-25° C. for 1 hour and then filtered onto a #2 Whatman™ paper filter. The cake was rinsed with water (50 mL, 5 vol.). The crude solids were collected and transferred back to a clean vessel.
[0164]Water (100 mL, 10 vol.) was added to the vessel containing the solids and the mixture was warmed to 35-40° C. for 2 hours, then filtered while warm...
example 2
Preparation of 6-bromo-2-chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
[0167]
Step A. Preparation of 5-bromo-2-chloro-6-cyclopentylamino-pyrimidine
[0168]To a vessel was added absolute ethanol (3000 mL, 3.0 vol) followed by 5-bromo-2,4-dichloropyrimidine (mw 227.87; 1000 g, 1.0 equiv.). Triethylamine (612 mL, 1.0 equiv.) was added, and then cyclopentylamine (mw 85.15; 520 mL, 1.2 equiv.) was added slowly over 2 hours to control the mild exotherm. After completion of cyclopentylamine addition, the reaction was seeded with 5-bromo-2-chloro-6-cyclopentylamino-pyrimidine (5 g, 0.5 wt %) to induce crystallization, if needed. The reaction was stirred at 25° C. for 2 hours.
[0169]Water (2500 mL, 2.5 vol) was added to the vessel at 20-25° C. at a rate of 30 mL / min. The mixture was cooled to 8-12° C. at 2° C. / min. The slurry was kept at 8-12° C. for 1 hour and then filtered onto a #2 Whatman™ paper filter. The cake was rinsed with n-heptane (2000 mL). The cake was reslurried with...
example 3
Preparation of 4-{6-[6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-2-ylamino]pyridin-3-yl}-piperazine-1-carboxylic acid tert-butyl ester
[0176]
[0177]A dry, nitrogen purged reactor was charged with tetrahydrofuran (900 mL, 15 mL / g). The batch temperature was set at 20° C. and agitation at 250 RPM was started. The reactor was charged with 4-(6-amino-pyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester (63.4 g, 0.2278 moles, 1.3 equiv.) and the mixture held at 20° C. for 30 min to dissolve the starting material. The reactor was charged with isopropylmagnesium chloride (93.9 g, 0.193 moles, 1st charge 1.1 eq) (2.0M in THF, 1.1 equiv.) by pump over 30 min. The batch was maintained at 20° C. for 40 min. The reactor was charged with 6-bromo-2-chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (60.1 g, 0.1755 moles, 1 eq.) all at once and rinsed with THF (50 mL rinse). An additional charge of isopropylmagnesium chloride (93.9 g, 0.193 moles, 1.1 eq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| primary particle size | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com