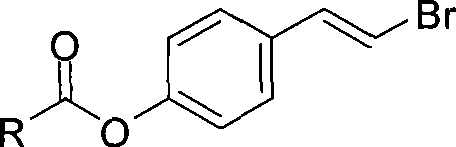
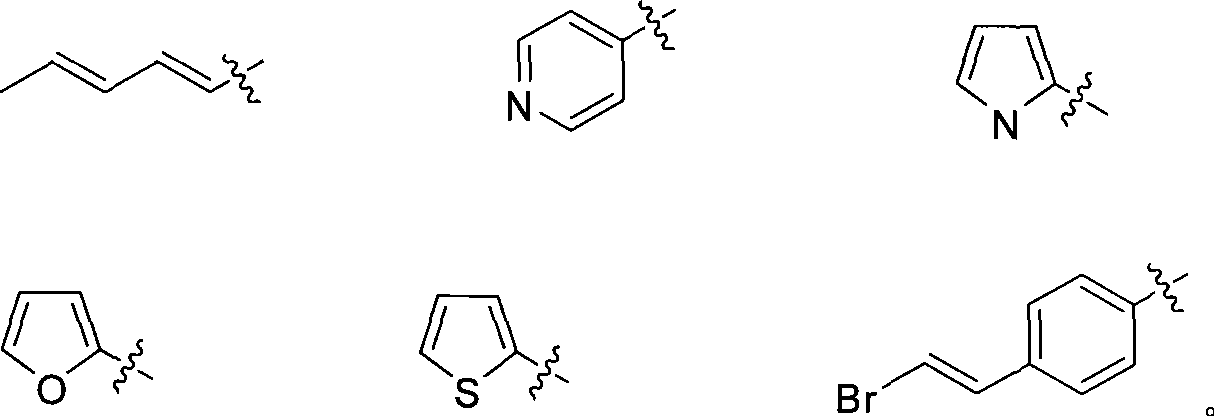
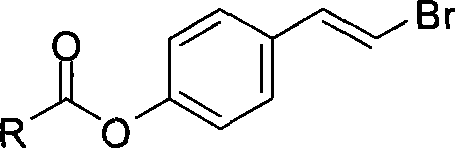
IDO restrainer containing (E)-4-(beta-bromo vinyl)benzoyloxy structure
A technology of phenoxyacyl and vinyl bromide, applied in organic chemistry, active ingredients of heterocyclic compounds, drug combinations, etc., can solve problems such as reducing the utilization of free serum tryptophan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of (E)-4-(beta-bromovinyl)phenol
[0027] The synthetic route is as follows:
[0028]
[0029] The first step: the preparation of 4-hydroxyacrylic acid
[0030] In a 50mL three-neck flask equipped with a reflux condenser and a thermometer, add 0.611g (5mmol) of 4-hydroxybenzaldehyde, 1.040g (10mmol) of malonic acid, 10mL of pyridine, and 3 drops of piperidine, and heat up to 80°C. The reaction was stirred for 6 to 8 hours, followed by TLC detection (ethyl acetate / n-hexane / acetic acid=10 / 20 / 1 as developing solvent). After the reaction, cool to room temperature, neutralize to pH=2 with 6M cold hydrochloric acid, filter, wash with ice water and ethyl acetate, P 2 o 5 Vacuum drying gave 0.700 g of light yellow powder, namely (E)-4-(β-bromovinyl)phenol, with a yield of 92%.
[0031] The second step: the preparation of (E)-3-(4-acetylphenyl)acrylic acid
[0032] In a 25mL egg-shaped bottle equipped with a calcium chloride drying tube, add...
Embodiment 2
[0037] Embodiment 2: Preparation of (E)-4-(β-bromovinyl)phenol 2-pyrrole ester (compound 1)
[0038] Add 10 mL (110 mmol) of benzene, 199 mg (1 mmol) of (E)-4-(β-bromovinyl) phenol, 117 mg (1.05 mmol) of 2-pyrrolic acid, 12 mg (0.1 mmol) of a 25 mL dry round bottom flask For p-dimethylaminopyridine (DMAP), stir magnetically at room temperature for 8 minutes, then add 206 mg (1 mmol) of N, N'-dicyclohexylcarbodiimide (DCC), and complete the reaction at room temperature for 24 hours. The solvent was evaporated under reduced pressure, and the residue was separated and purified by column chromatography using ethyl acetate:petroleum ether=1:5-1:9 as the eluent to obtain (E)-4-(β-bromovinyl)phenol 2 - pyrrole ester 285 mg, the yield is 98%. The reaction and product characterization data are as follows:
[0039]
[0040] Light yellow solid; mp130.6-132.7℃.
[0041] IR (KBr): 1719, 935, 751cm -1 .
[0042] 1 H NMR (300MHz, CDCl 3 ): δ=6.36-6.37 (1H, m), 6.75 (1H, d, 14.4Hz),...
Embodiment 3
[0044] Embodiment 3: Preparation of (E)-4-(β-bromovinyl)phenol 2-furanoic acid ester (compound 2)
[0045] Add 12mL (130mmol) of benzene, 199mg (1mmol) of (E)-4-(β-bromovinyl)phenol, 123mg (1.1mmol) of 2-furanoic acid, 61mg (0.5mmol) For p-dimethylaminopyridine (DMAP), 227 mg (1.1 mmol) of N, N'-dicyclohexylcarbodiimide (DCC) was added after magnetic stirring at room temperature for 10 minutes, and the reaction was completed at room temperature for 22 hours. The solvent was evaporated under reduced pressure, and the residue was separated and purified by column chromatography using ethyl acetate:petroleum ether=1:6-1:10 as the eluent to obtain (E)-4-(β-bromovinyl)phenol 2 - Furanate 270mg, the yield is 92%. The reaction and product characterization data are as follows:
[0046]
[0047] White solid; mp100.6-100.8℃.
[0048] IR (KBr): 1734, 930, 762cm -1 .
[0049] 1 H NMR (300MHz, CDCl 3 ): δ = 6.60-6.61 (1H, m), 6.76 (1H, d, J = 14.1Hz), 7.11 (1H, d, J = 14.1Hz), 7.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com