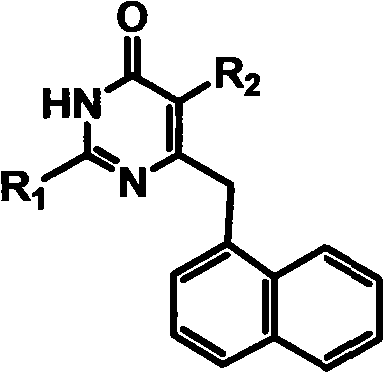
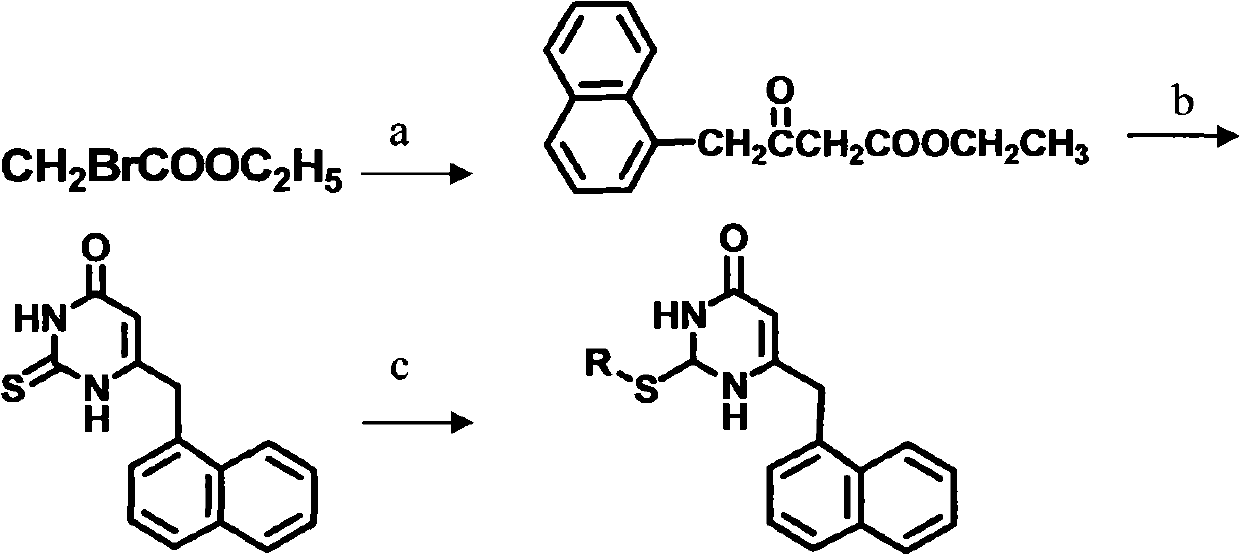
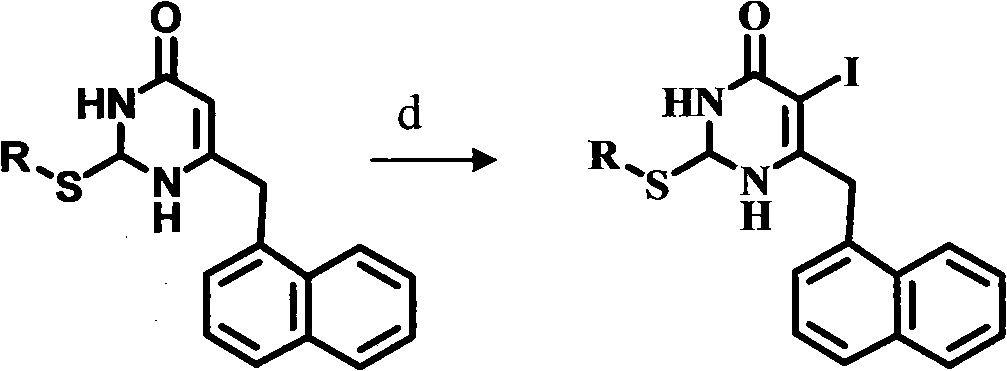
Preparation and application of novel chiral 3,4-dihydro-2-alkoxyl-6-benzyl-4-oxopyrimidine (S-DABO) human immunodeficiency virus (HIV)-1 reverse transcriptase inhibitor
A CH3, C6H5 technology, applied in antiviral agents, organic chemistry, etc., can solve problems such as drug-resistant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Compound 1: Ethyl 3-oxo-4-(1-naphthyl)butanoate
[0027] The activated zinc powder (18g, with 3N hydrochloric acid, H 2 (0, absolute ethanol, anhydrous ether washed three times, then vacuum-dried and dried), suspended in dry THF (125ml) and heated to 85°C for reflux, 20 drops of ethyl bromoacetate was added dropwise, after 45 minutes (the solution became (green) was added 1-naphthaleneacetonitrile (2.004g, 12mmol) in one go, then ethyl bromoacetate (3.3ml, 30mmol) was slowly added dropwise, and the dropwise addition was completed after 1 hour, and then refluxed for 10 minutes. Dilute with THF (3 × 125ml), add potassium carbonate (50%, 54ml) aqueous solution and stir rapidly for 45 minutes, let stand to separate layers, pour out the upper organic solvent, wash the remaining aqueous phase with THF (2 × 100ml), and combine the organic phases Then add hydrochloric acid (10%, 50ml) and stir at room temperature for 45 minutes, 3 When the pH value is adjusted to ...
Embodiment 2
[0191] Embodiment 2 carries out the biological activity evaluation of HIV-1 reverse transcriptase to the synthesized compound
[0192] One experimental principle
[0193] 1. Reverse transcriptase:
[0194] Reverse transcriptase is a DNA polymerase that synthesizes double-stranded DNA using single-stranded RNA as a template. It is a multi-functional enzyme, which has the activities of synthesizing polymerase and ribonuclease H respectively using RNA or DNA as a template.
[0195] 2. Nucleotide microplate covalently cross-linked
[0196] Nucleic acid molecular hybridization can be divided into two types: solid-phase hybridization and liquid-phase hybridization. Solid-phase hybridization is to immobilize one nucleic acid participating in the reaction on a solid support, and the other nucleic acid reaction chain is free in the solution. In this experiment, oligo(dT) will be used as the primer 15 After phosphorylation at the 5' end of NUNC, covalent cross-linking occurs with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com