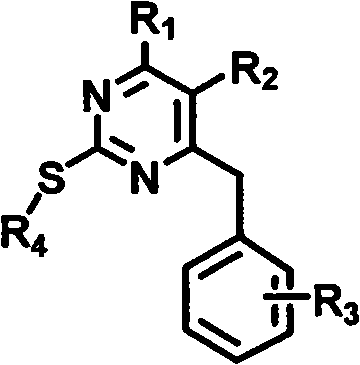
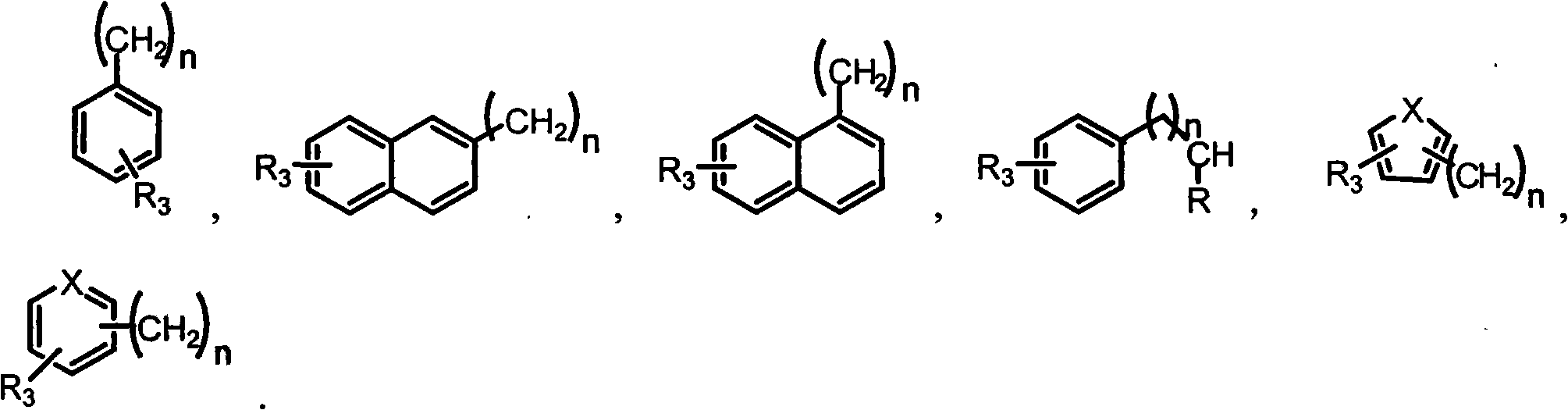
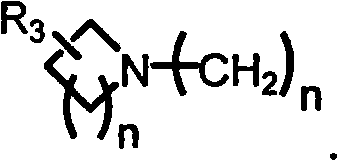Preparation method and use of novel S-DABO HIV-1 reverse transcriptase inhibitor
A technology of HIV-1 and reagents, applied in the medical field, can solve problems such as drug-resistant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Compound 1: Ethyl 3-oxo-4-phenylbutyrate
[0025] Ethyl 3-oxo-4-phenylbutyrate
[0026] The activated Zn powder (18g, was washed with 3N hydrochloric acid, H 2 O, Anhydrous EtOH, Anhydrous Et 2 O washed three times, then vacuum dried), suspended in dry THF (125ml) and heated to reflux, dropwise into 20 drops of BrCH 2 COOEt, after 45 minutes (green substance appears in the solution), add C in one go 6 h 5 CH 2 CN (3.12ml, 27mmol), BrCH was added dropwise 2 COOEt (6.6ml, 60mmol) was added dropwise after 1 hour and then refluxed for 10 minutes. Dilute with THF (125×3ml), add K 2 CO 3 (50%, 54ml) aqueous solution was rapidly stirred for 45 minutes, and the layers were left to stand, and the upper organic solvent was poured out, and the remaining residue was washed with THF (2×100ml), and the combined organic phases were added with hydrochloric acid (10%, 50ml) and stirred at room temperature for 45 minutes with NaHCO 3 Adjust PH≈7 and let it stand for stratificat...
Embodiment 2
[0029] Compound 2: Ethyl 2-methyl-3-oxo-4-phenylbutyrate
[0030] Ethyl-2-methyl-3-oxo-Phenylbutanoate
[0031] The activated Zn powder (13g, with 3N hydrochloric acid, H 2 O, Anhydrous EtOH, Anhydrous Et 2 O washed three times, then vacuum dried), suspended in dry THF (45ml) and heated to reflux, dripped 20 drops of ethyl bromoacetate, and after 45 minutes (the solution appeared green substance) added benzonitrile (1.3 ml, 10mmol), dropwise added BrCH (CH 3 )COOEt (5.5ml, 50mmol), after 1 hour, the dropwise addition was completed and then refluxed for 10 minutes. Dilute with THF (45×3ml), add K 2 CO 3 (50%, 15ml) aqueous solution was stirred rapidly for 45 minutes, and the layers were left to stand, and the upper organic solvent was poured out, and the remaining residue was washed with THF (2×50ml), and the combined organic phases were added with hydrochloric acid (10%, 12ml) and stirred at room temperature for 45 minutes with NaHCO 3 Adjust PH≈7 and let it stand for s...
Embodiment 3
[0034] Compound 3: 6-Benzylthiouracil
[0035] 6-benzyl-2-thioxo-2, 3-dihydropyrimidin-4(1H)-one
[0036] Sodium metal (4g, 174mmol) was dissolved in anhydrous EtOH (90ml), SO(NH 2 ) 2 (9.28g, 120mmol), and compound 1 (1.64g, 8.0mmol), the reaction mixture was heated to reflux for 6 hours. Rotate under reduced pressure at 40-50°C until almost completely dry, and dissolve the residue in water (80ml). Concentrated hydrochloric acid (14ml) was added to precipitate a precipitate, and HOAC was added to adjust until pH ≈ 4. The precipitate was filtered out with a suction funnel, washed with 10% EtOH solution, and dried to obtain 1.53 g of a white solid with a yield of 86.5%.
[0037] MS (ESI) m / z: 219 [M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com