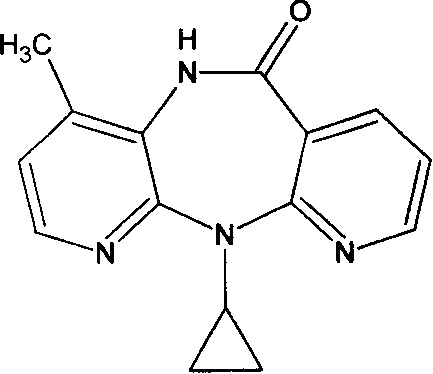
Viramune slice and its preparing method thereof
A technology of nevirapine and povidone, which is applied in the field of nevirapine tablets and its preparation, can solve the problems of high cost, high cost, and low yield, and achieve the effects of high yield, low cost, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of nevirapine tablet of the present invention is carried out according to the following steps:
[0019] (1) Get the nevirapine, the lactose, the microcrystalline cellulose, and the carboxymethyl starch sodium in the weight ratio, respectively pulverize them and pass through an 80-mesh sieve, then mix them evenly and pass through a 60-mesh sieve to obtain a mixed powder ;
[0020] (2) Get the povidone K30 in the weight ratio, add pure water to form a solution;
[0021] (3) Using the povidone K30 solution as a binder, the mixed powder obtained in step (1) is made into a soft material (the soft material should be held into agglomerates, and it will disperse when touched), and passed through a 20-mesh sieve to obtain a wet The granules are dried, and then passed through a 18-mesh sieve to make dry granules;
[0022] (4) cross 100 mesh sieves after getting the described magnesium stearate pulverization of described weight proportion;
[0023] (5) th...
Embodiment 1
[0027] In the present embodiment, the weight of each raw material is respectively:
[0028] Nevirapine 200g, lactose 120g, microcrystalline cellulose 50g, sodium starch glycolate 15g, micropowder silica gel 4g, magnesium stearate 3g, povidone K30 2g. 1000 tablets of nevirapine can be prepared, and each tablet contains 200 mg of active ingredient nevirapine.
[0029] Dosage and dosage:
[0030] oral.
[0031] During the first 14 days, the normal dose (the dose of the active ingredient nevirapine) is 200 mg once a day (this lead-in period should reduce the incidence of rash). Usage after the lead-in period, 2 times a day, 200mg each time. (The once-daily dosing must be strictly adhered to during the 14-day lead-in period before escalating to twice-daily dosing.) If treatment is interrupted for more than 7 days, the starting once-daily dose should be resumed when treatment is reinitiated14 days before increasing the dose to twice a day.
Embodiment 2
[0033] In the present embodiment, the weight of each raw material is respectively:
[0034] Nevirapine 200g, lactose 150g, microcrystalline cellulose 70g, sodium starch glycolate 20g, micropowder silica gel 5g, magnesium stearate 4g, povidone K30 3g. 1000 tablets of nevirapine can be prepared, each tablet contains 200 mg of active ingredient nevirapine, and each tablet contains 200 mg of active ingredient nevirapine.
[0035] All the other are the same as embodiment one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com