Biomarker for forecasting severe drug-induced cutaneous adverse reaction of child patient and application
A biomarker and adverse reaction technology, applied in the field of biomarkers to predict severe drug-induced skin adverse reactions in children, can solve the problems of limited predictive ability of adult SCARs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
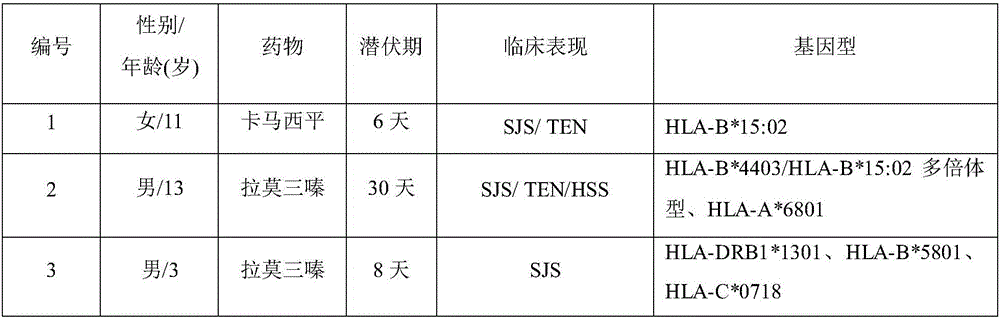
[0015] 1. Collect peripheral venous blood samples or oral exfoliated cells.
[0016] 2. Genomic DNA extraction.
[0017] 3. For different drugs β-lactam antibiotics (penicillin, cephalosporin), carbamazepine, lamotrigine, oxcarbazepine, phenytoin, allopurinol, nevirapine, abacavir, methazolamide, Dapsone selects different genetic testing kits.
[0018] (1) β-lactam antibiotics (penicillins, cephalosporins): including IL-13R130Q, IL-4RA I50V, IL-4RA S478P, IL-4RA Q551R, IL-13 1055C>T, IL-13 1055C>T , IL-4RA Q551R, IL-4RαIle75Val, IL-10-819C>T, IL-10-592C>A, IL-4RαQ576R AA, IL-4RαI75V AA, IL-4RαQ576 / I75, IL-18-607A / C, A genetic detection kit for one or more combinations or haplotypes in IL-18-137G / C polymorphism.
[0019] (2) Carbamazepine: a genetic detection kit containing one or more combinations or haplotypes of HLA-B*15:02, HLA-B*15:11, and HLA-A*31:01 polymorphisms .
[0020] (3) Lamotrigine: including HLA-B*4403, HLA-B*15:02, HLA-A*6801, HLA-B*5801, HLA-C*0718, HLA-D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com