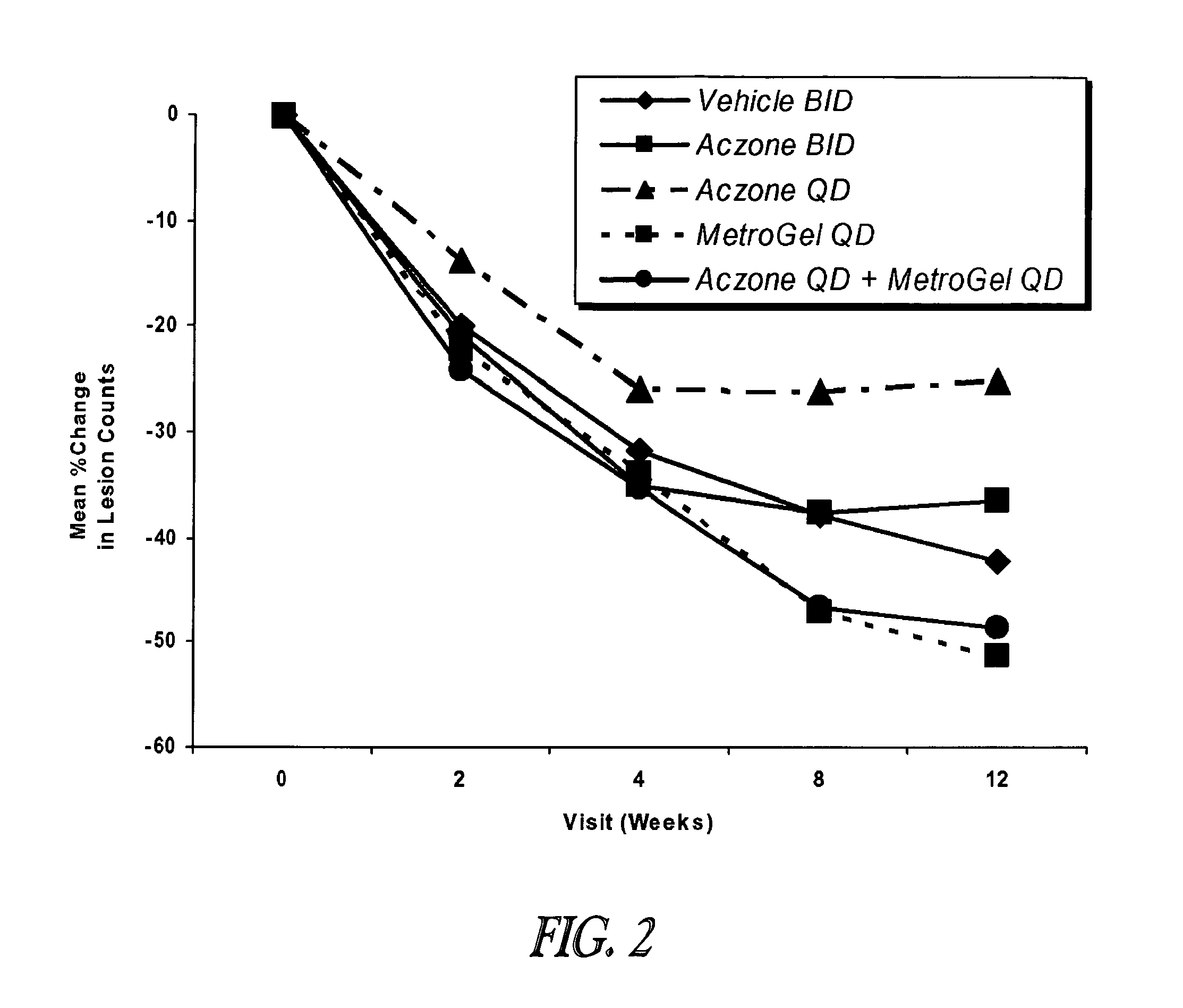
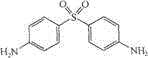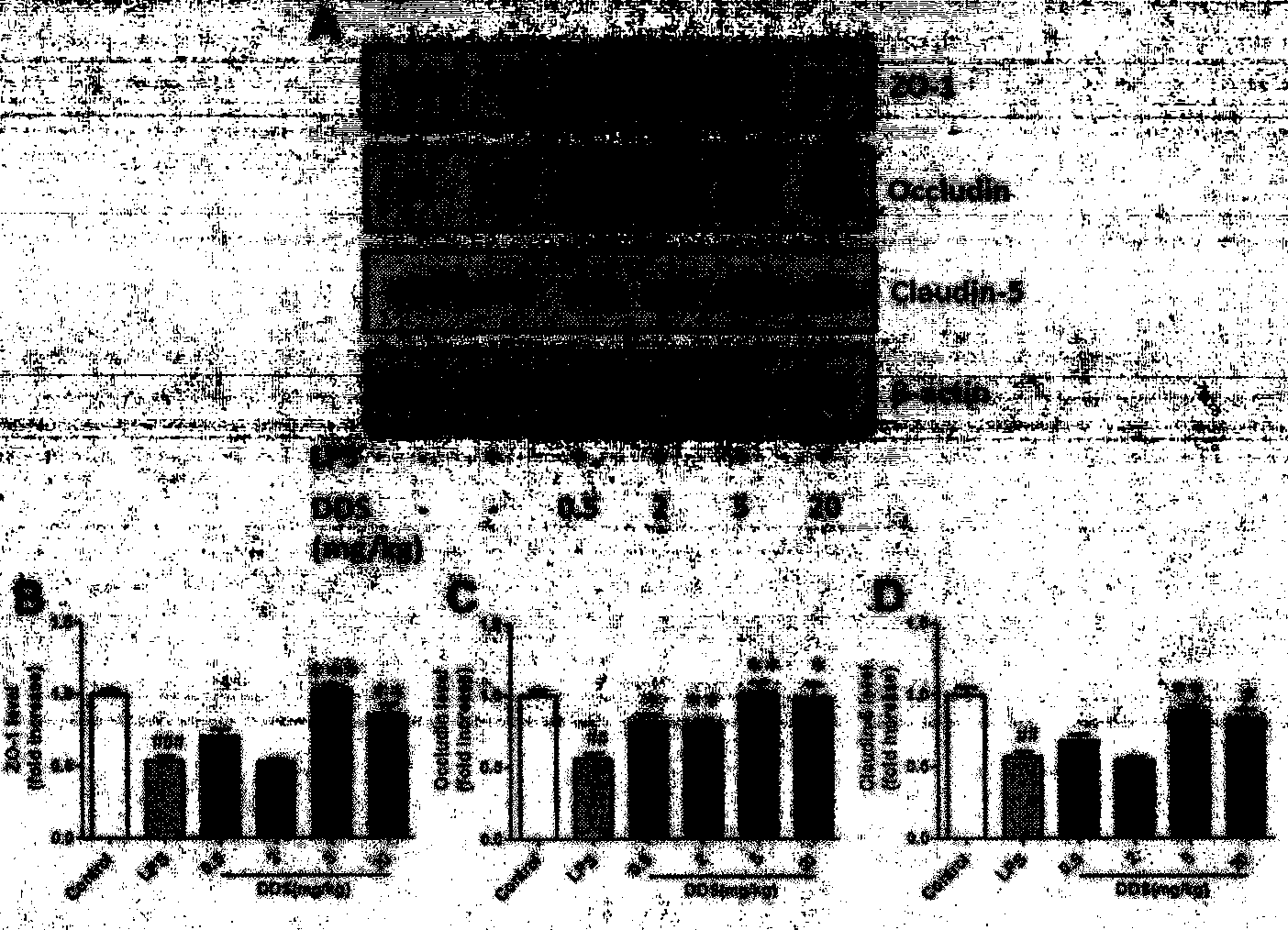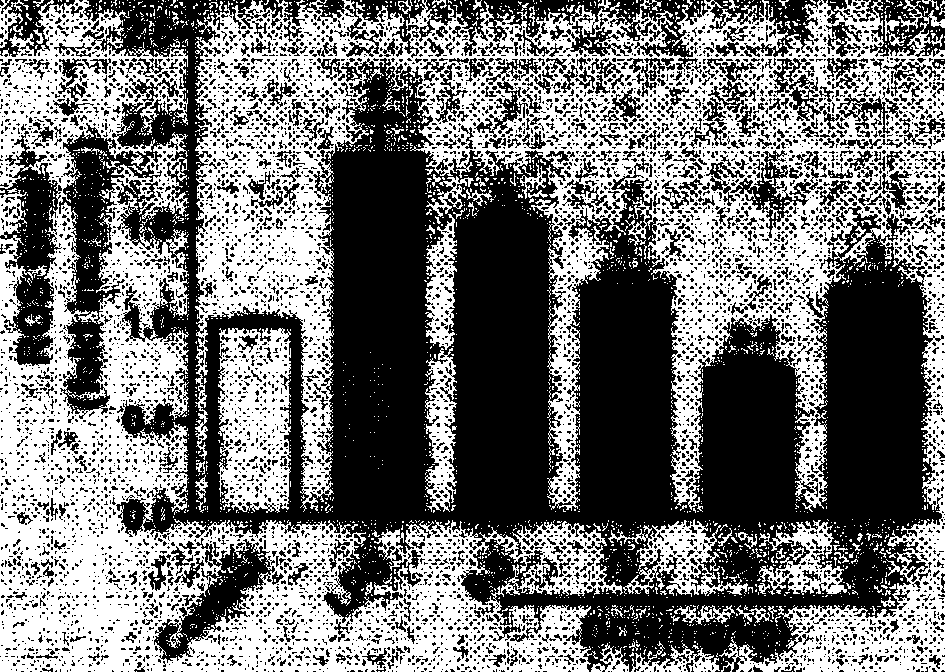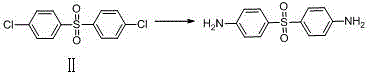Patents
Literature
53 results about "Dapsone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
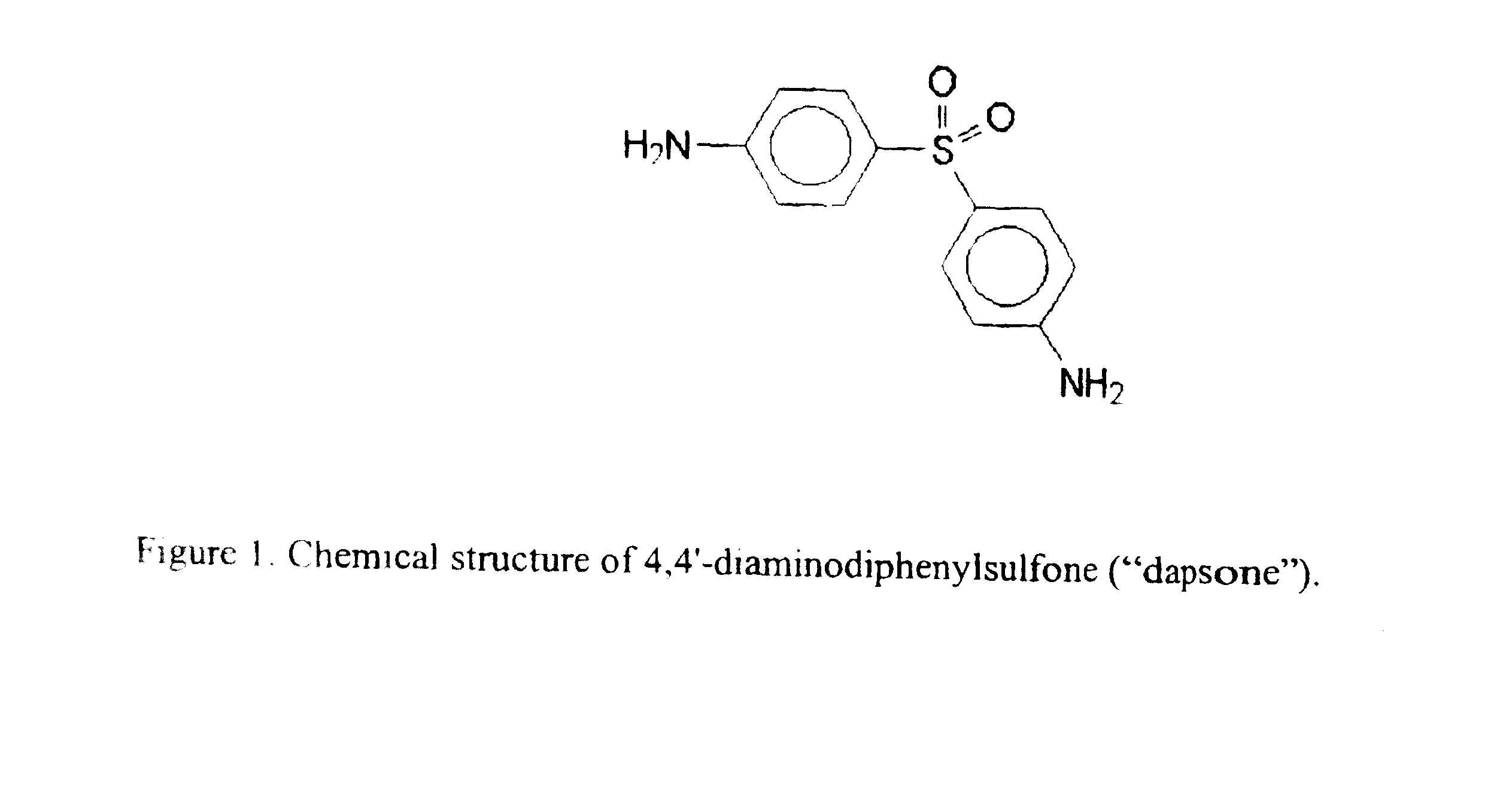
This medication is used to treat a certain type of skin disorder (dermatitis herpetiformis). It is also used with other drugs to treat Hansen's disease.
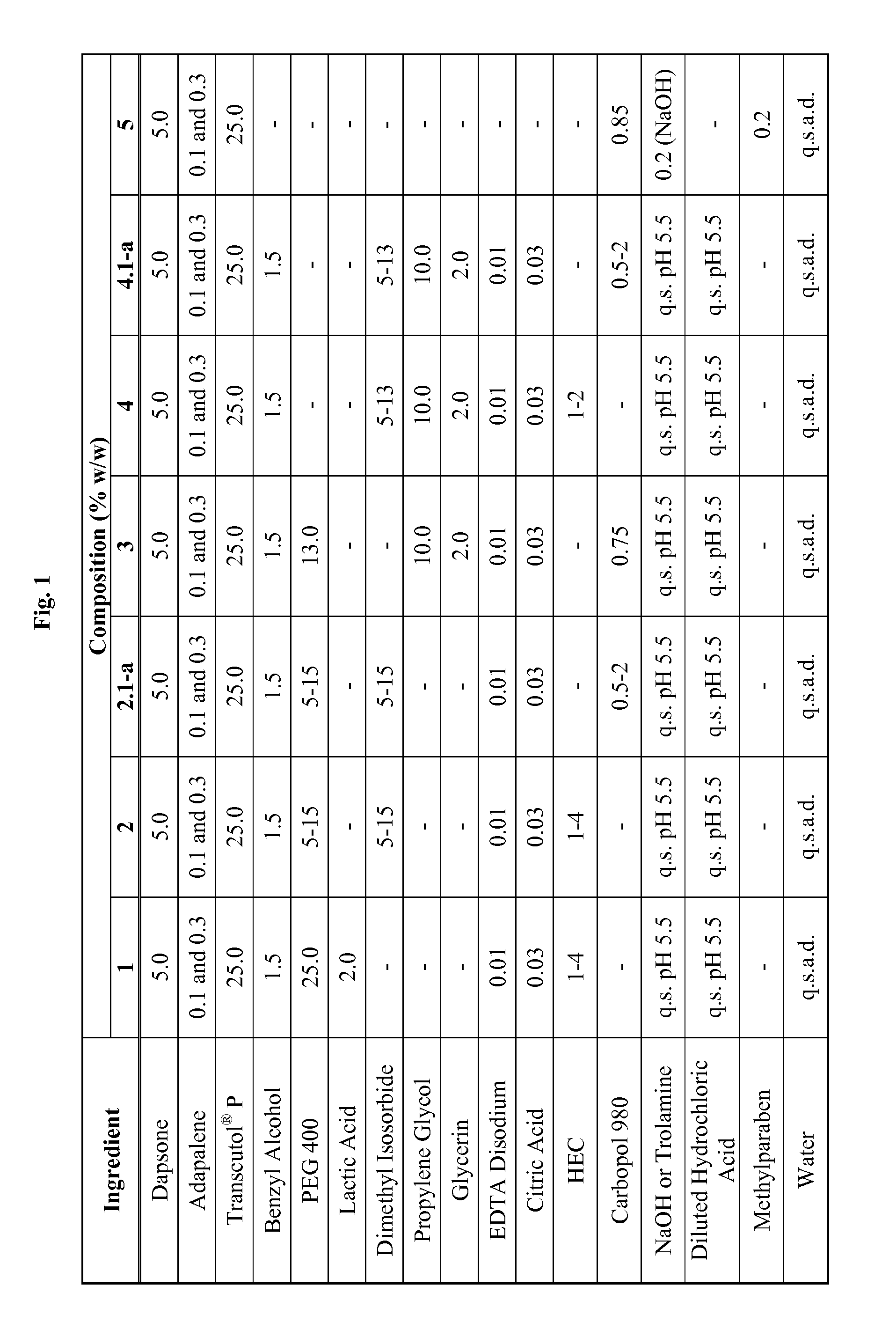
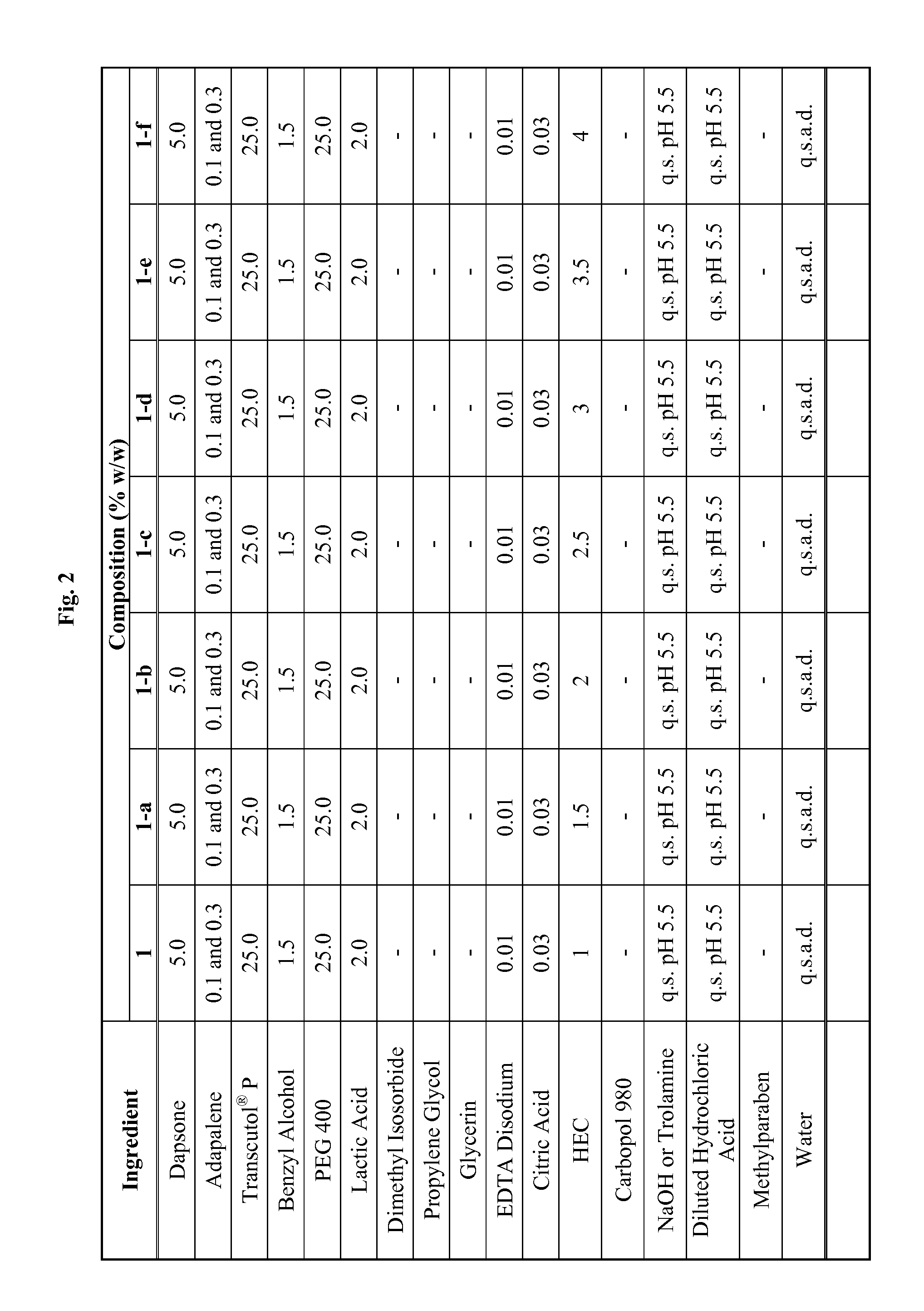
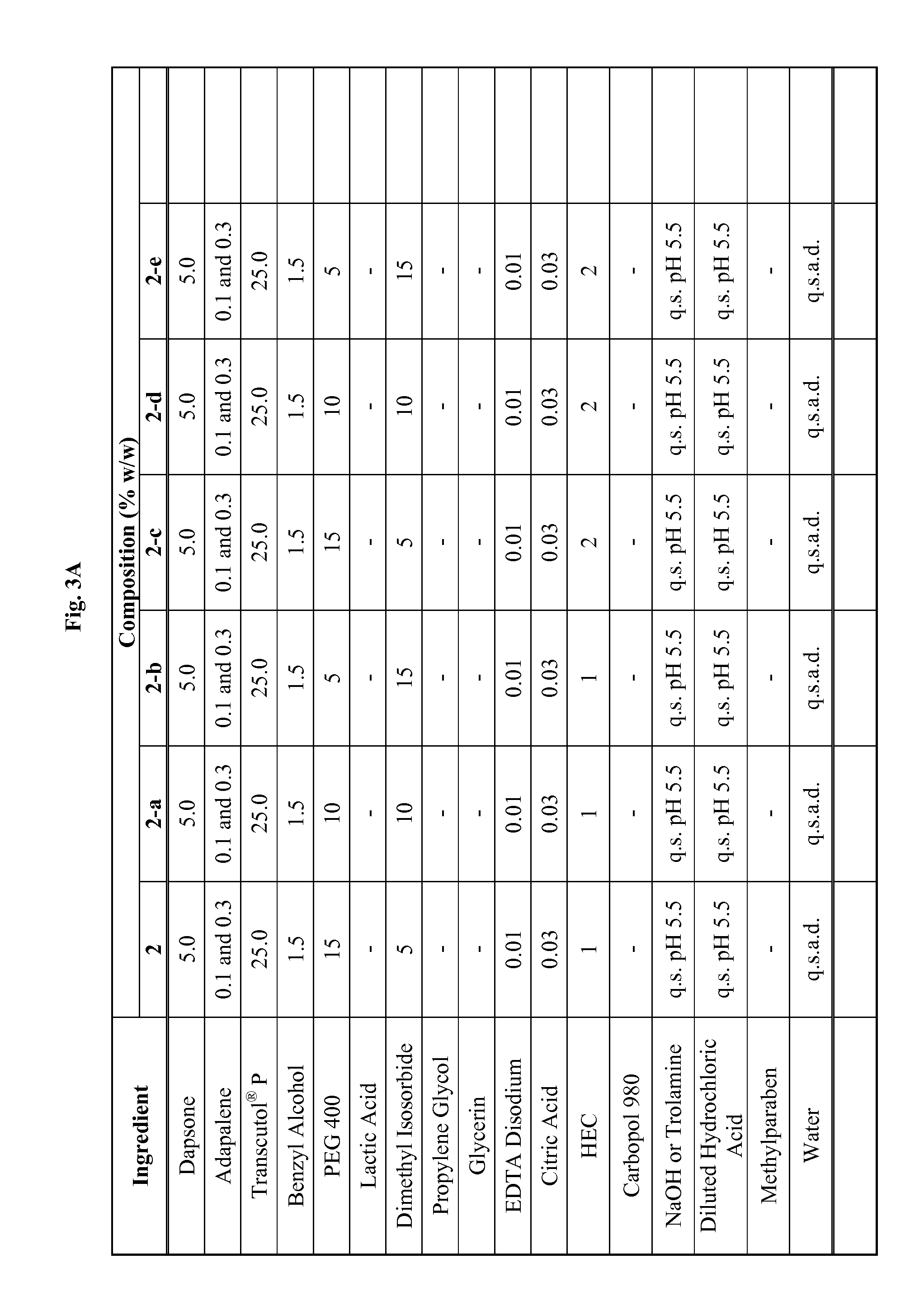
Emulsive composition containing Dapsone
InactiveUS20060204526A1Stabilizes emulsive compositionAvoid separationAntibacterial agentsBiocideSolubilityEmulsion
The present invention relates to a topical, emulsive composition containing Dapsone or its derivative. The inventive composition incorporates emollients and Dapsone or its derivative in a stable emulsion. The stability is achieved through the use of a combination of certain surfactant mixtures and an enhancer providing solubility of the Dapsone.
Owner:ALLERGAN INC
Topical compositions for the treatment of chronic wounds
InactiveUS20060286108A1Avoid actionLow cytotoxicityBiocideAntibody ingredientsTace inhibitorEfalizumab
Methods for treating chronic wounds in a human are described by topically administering a dermatological composition comprising a TNF antagonist, a TACE inhibitor, a neutrophil antagonist, or a combination of a TNF antagonist and / or TACE inhibitor and a neutrophil antagonist. The TNF antagonist administered includes alefacept, efalizumab, etanercept, adalimumab, and onercept, while the neutrophil antagonist administered includes dapsone, colchicine, its analogs and prodrugs. The combination of TNF-antagonist and neutrophil antagonist administered includes sulfapyridine, sulfasalazine, mesalamine, and derivatives and prodrugs thereof. The topical compositions can be formulated to include the one or more of the antagonists in dissolved, semi-dissolved, and micro-particulate states.
Owner:BELL DERMATOLOGICS
Treatment methods with peroxides and tertiary amines
InactiveUS20070244195A1Good curative effectBiocidePeroxide active ingredientsBenzoyl peroxideSide chain
This invention relates to methods of increasing the efficacy of peroxides such as benzoyl peroxide in the treatment of skin conditions such as acne. In a preferred embodiment, the invention relates to methods of increasing radicals formed by peroxides on / in the skin, more specifically near / in the comedone, for topical use in dermatology. In a specific embodiment, the invention relates to the use of transitional metals such as Cu(l) and ferrous ions to increase the efficacy of peroxides such as benzoyl peroxide. In another embodiment, the invention relates to a method by which a peroxide such as benzoyl peroxide and its activator are added to the skin surface at the same time. In another embodiment, the invention relates to the use of a more soluble form of peroxide such as benzoyl peroxide to increase its efficacy. In another embodiment, the invention relates to the addition of a side chain to a peroxide such as benzoyl peroxide so that it is activated by light. In a further embodiment, the invention relates to the addition of a tertiary amine to a peroxide such as benzoyl peroxide at the time of skin application, to improve the efficacy of the peroxide. In another embodiment, the invention relates to the addition of dapsone or other material to a peroxide such as benzoyl peroxide to improve its efficacy.
Owner:BURKHART CRAIG N +1
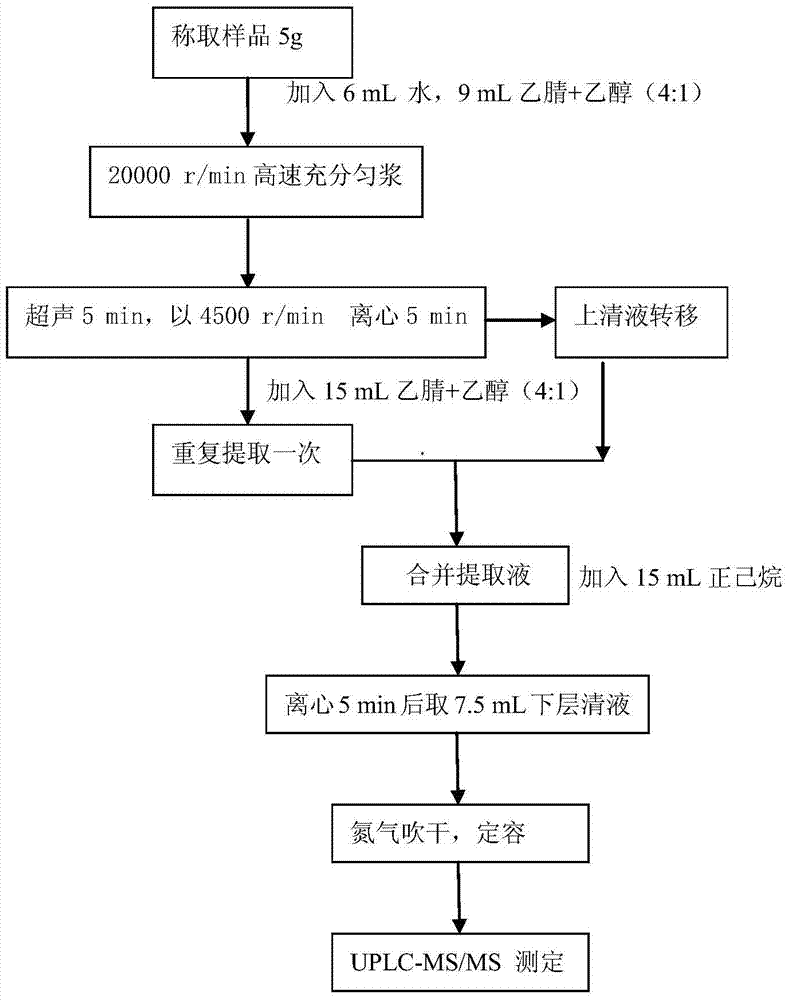
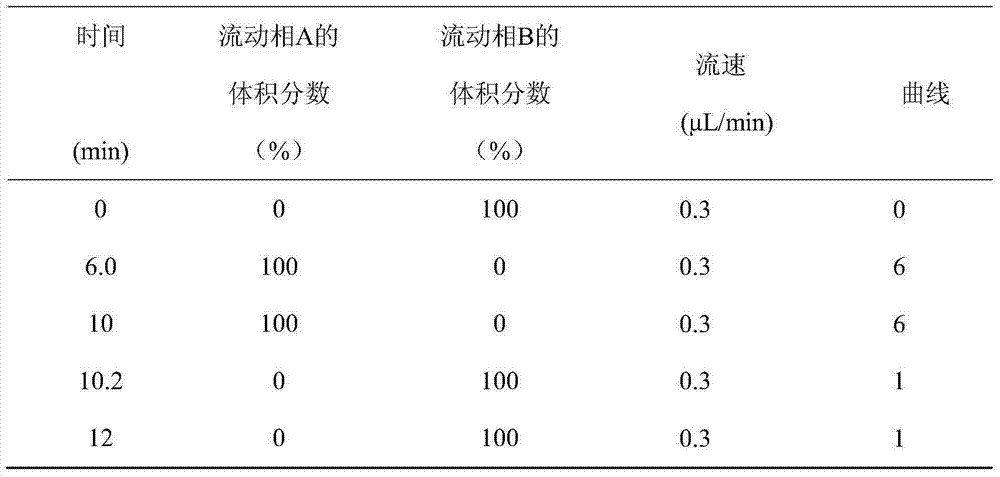
Method for simultaneously analyzing and detecting residual veterinary drug compositions in animal tissue
InactiveCN103713056AImprove linearitySimple processing and analysisComponent separationSulfur drugSulfanilamide
A disclosed method for simultaneously analyzing and detecting residual veterinary drug compositions in animal tissue comprises: performing homogenate on an animal tissue sample by 50% acetonitrile and ethanol, performing ultrasonic extraction and hexane purifying, and performing further precipitation by acetonitrile and ethanol, concentrating, utilizing UPLC-MS / MS to perform multi-reaction monitoring (MRM) determination under the negative ion mode, and quantifying according to a standard curve and an external standard method, detecting and calculating to obtain the content of 46 residual veterinary drug compositions in animal tissue. The method is applicable to present commonly-used veterinary drugs such as 18 kinds of quinolone drugs, 22 kinds of sulfanilamide drugs, dapsone, phenylethanolamine A, amoxicillin, adamantanamine, rimantadine, ethoxyquin and the like, and is capable of performing one-step simultaneous rapid accurate detection, and the application scope of the method is enlarged.
Owner:INSPECTION & QUARANTINE TECH CENT OF NINGBO ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Combination of dapsone with other Anti-acne agents
InactiveUS20110117182A1Good curative effectCosmetic preparationsBiocideEffective treatmentActive ingredient
A composition suitable for topical application that contains at least two active ingredients, one of these being dapsone and one selected from the group consisting of adapalene, tazarotene and treinion for the effective treatment of acne and other dermatological conditions.
Owner:ALLERGAN INC
Protein chip for detecting multiple veterinary drug residues and kit thereof
InactiveCN101782580AAvoid potential hazardsAvoid pollutionMaterial analysis by observing effect on chemical indicatorVeterinary DrugsDiethylstilbestrol
The invention discloses a protein chip for detecting multiple veterinary drug residues and a kit thereof. The protein chip comprises a substrate and dot coatings of veterinary drug antibody, wherein the dot coatings are distributed in an array type, the substrate is a glass substrate or a membrane substrate; and the dot coatings of the veterinary drug antibody refer to eight types of veterinary drug antibodies, i.e. an anti-chloramphenicol antibody, an anti-chlorpromazine antibody, an anti-colchicin antibody, an anti-dapsone antibody, an anti-stilboestrol antibody, an anti-dimetridazole antibody, an anti-furazolidone antibody and an anti-metronidazole antibody which are uniformly distributed and in dot lattice on the substrate. The protein chip can obtain the reaction results with multiple indexes by only one-step reaction and can be suitable for simultaneously detecting eight veterinary drugs, i.e. chloramphenicol, chlorpromazine, colchicin, dapsone, stilboestrol, dimetridazole, furazolidone and metronidazole which are contained in a sample, thereby ensuring the safety and the sanitation of a large quantity of meat products.
Owner:上海裕隆生物科技有限公司
Topical dapsone for the treatment of acne
The present invention relates to a method of treating acne by topically applying a dermatological composition comprising dapsone. In addition to inflammatory lesions, the composition also treats non-inflammatory acne. The composition is formulated to include dapsone in a both a dissolved and microparticulate state.
Owner:ALLERGAN INC
Galenical preparations of dapsone and related sulphones, and method of therapeutic and preventative treatment of disease
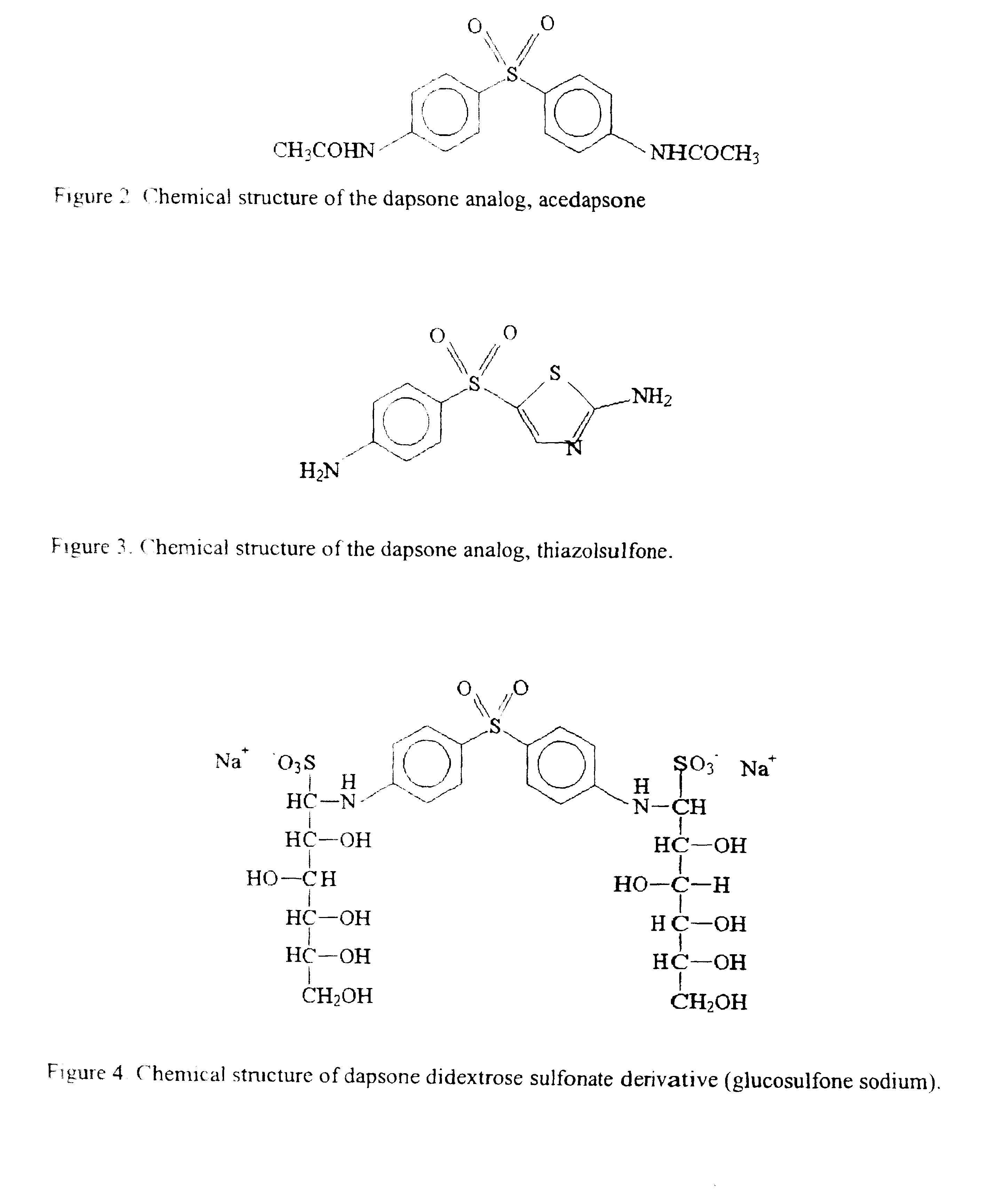
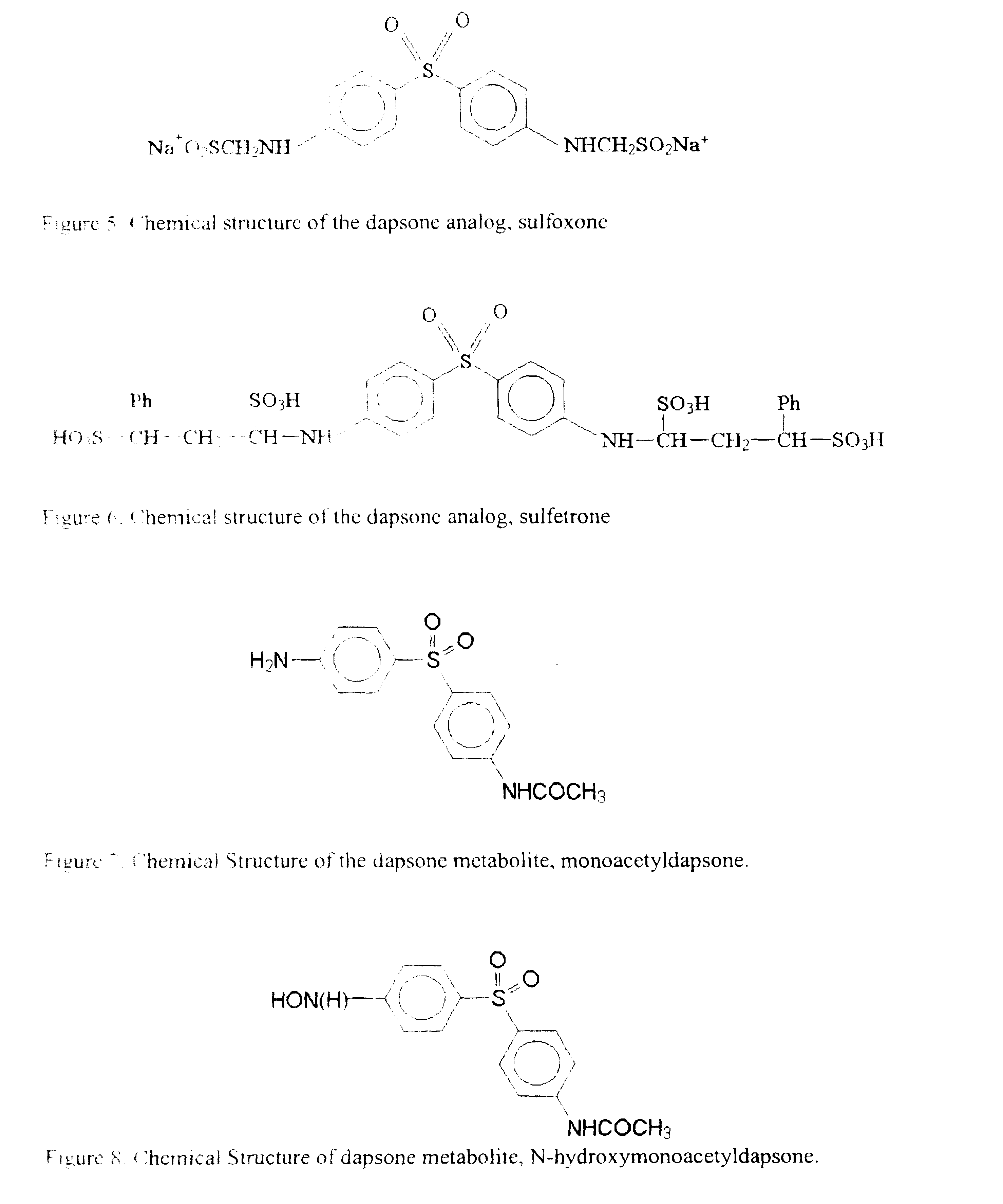
Dapsone and related sulfones are known to have therapeutic activity against leprosy, dermatitis herpetiformis, actinomycotic mycetoma, asthma, malaria, rheumatoid arthritis, Kaposiís sarcoma, pneumocystis carinií (pneumonia), subcorneal pustular dermatosis and cystic acne, in patients in need of such therapy. These sulfones are also known to have therapeutic activity against memory loss in patients in need of such therapy, including patients suffering from Alzheimer's disease and related neurodegenerative disorders. It has now been found that new, modified-release formulations of dapsone and related sulfones may also be used that decrease side effects and increase effectiveness of the drugs. New methods are disclosed utilizing certain formulations of dapsone and related sulfones that improve the therapeutic index of said drugs. Side effects of these drugs are known to those skilled in the art and include, but are not restricted to anorexia, psychosis, agranulocytosis, peripheral neuritis, hemolysis, methemoglobinemia, nausea, vomiting, headache, dizziness, tachycardia, nervousness, insomnia and skin disorders. Modified-release (as defined herein) formulations of dapsone have now been found to avoid some or all of these side effects, and to have more efficacy on potency.
Owner:IMMUNE NETWORK
Protectant for UV-induced skin damage
The present invention provides a method for protecting against UV radiation-induced skin damage. Specifically, compositions including dapsone are administered to provide UV protection. The dapsone compositions may be administered orally, or by other parenteral routes, such as topically, transdermally, by inhalation, and the like.
Owner:ALLERGAN INC
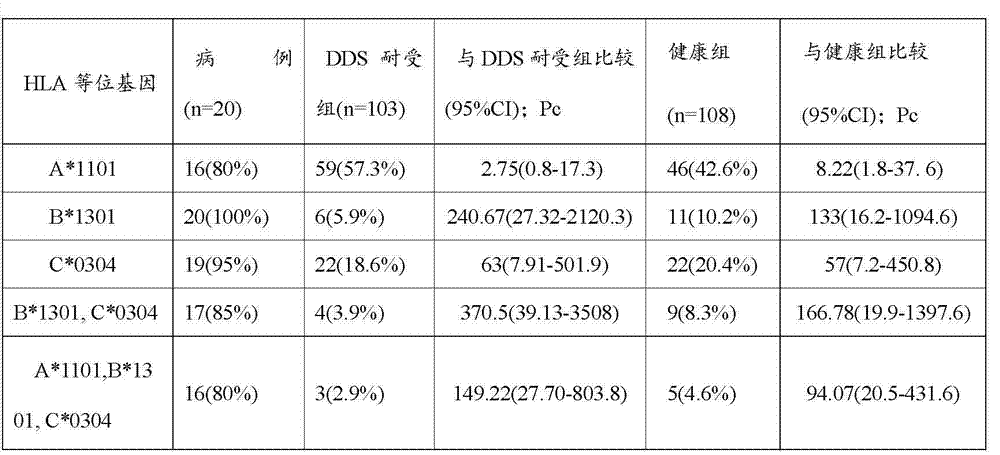
Use of hla-b*1301 allele
The present invention discloses uses of a HLA-B*1301 allele, comprising: 1) a use of a substance for detecting whether a person has the HLA-B*1301 allele in preparation of a product for evaluating a risk of adverse drug reactions in response to dapsone in the person; 2) a method for detecting or evaluating a risk of adverse drug reaction in response to dapsone in a person, comprising detecting whether the person has the HLA-B*1301 allele, wherein, a person with LA-B*1301 allele suffers a higher risk of adverse drug reaction upon being administered dapsone, as compared with a person without HLA-B*1301 allele, and a person with LA-B*1301 alleles at both chromosomes of a pair of homologous chromosomes suffers a higher risk of adverse drug reaction upon being administered dapsone, as compared with a person with HLA-B*1301 allele at only one of a pair of homologous chromosomes.
Owner:SHANDONG PROVINCIAL INST OF DERMATOLOGY & VENEREOLOGY
Biomarker for forecasting severe drug-induced cutaneous adverse reaction of child patient and application
The invention discloses a biomarker for forecasting severe drug-induced cutaneous adverse reaction of a child patient and an application. The biomarker is capable of forecasting the risk of severe cutaneous adverse reaction of the child patient using beta-lactam antibiotics such as penicillin, cephalosporin, carbamazepine, lamotrigine, oxcarbazepine, phenytoin, allopurinol, nevirapine, abacavir, methazolamide and dapsone.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Preparation method for magnetic chitosan dapsone surface imprinting adsorbing material
InactiveCN103816874AImprove adsorption capacityNo phenomenon of local aggregationOther chemical processesAlkali metal oxides/hydroxidesEpoxyCross linker
The invention discloses a preparation method for a magnetic chitosan dapsone surface imprinting adsorbing material, particularly relates to a surface imprinting technology which builds molecule recognition sites on the surface of a substrate material. The preparation method comprises the following steps: modified chitosan and Fe3O4 particles are combined to prepare magnetic chitosan, the magnetic chitosan is further adopted, the dapsone is used as template molecules, epoxy chloropropane is used as a cross-linking agent, and then the water-insoluble acid-insoluble magnetic chitosan dapsone surface imprinting adsorbing material is prepared. The adsorbing material prepared through the preparation method disclosed by the invention has the advantages that the selectivity is high, the adsorption capacity is large, the separation operation is easy, and the reusability is good; according to the invention, the powerful material support for a novel chemical sensor, which is high in selectivity and used for measuring dapsone is provided, a novel way for separating and recycling the dapsone from waste water is developed, and effective methods for monitoring and processing environmental pollutants are provided.
Owner:UNIV OF JINAN
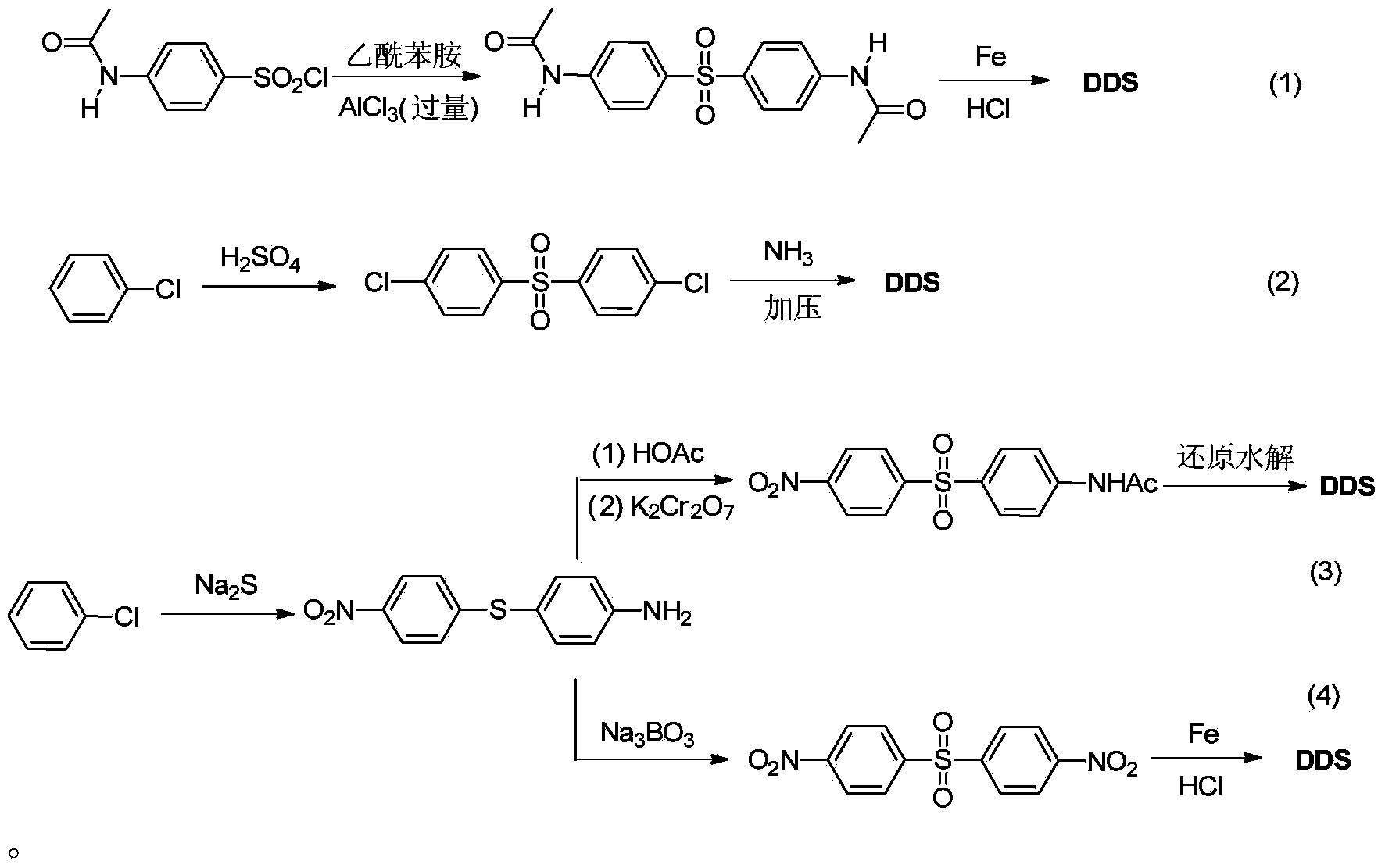
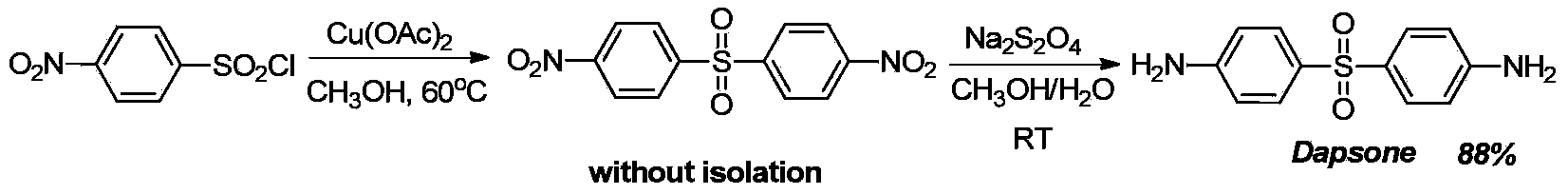
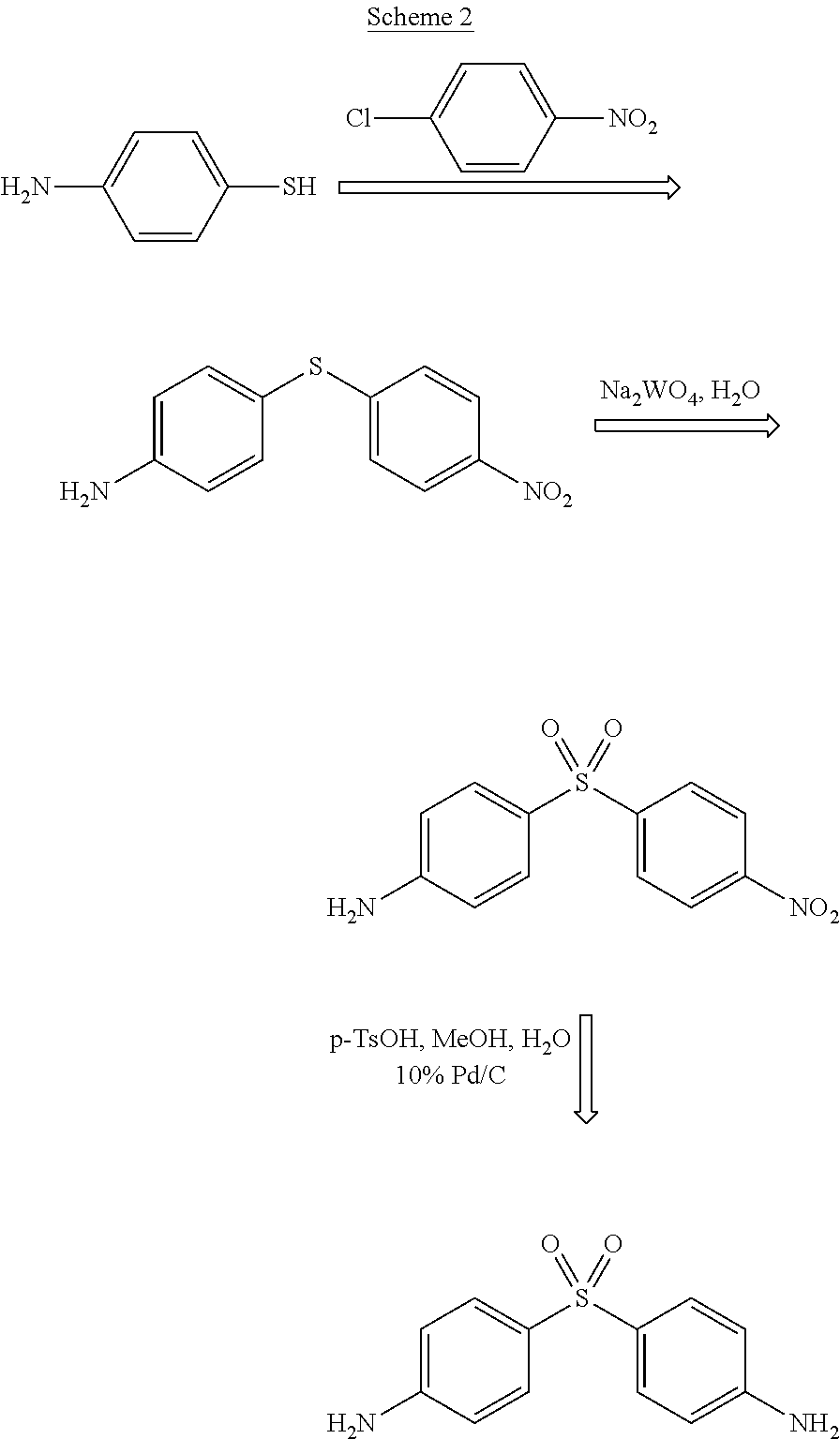
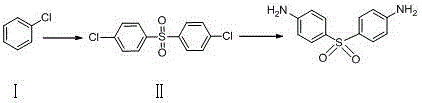
Preparation method of dapsone
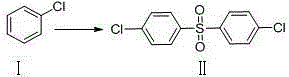
ActiveCN103641753ANo protectionImprove practicalityOrganic chemistryOrganic compound preparationP-nitrobenzenesulfonyl chlorideSolvent
The invention discloses a preparation method of dapsone, and more in particular relates to a method for preparing dapsone by a one-pot method. The preparation method of dapsone is characterized by comprising the steps of: by taking nitrobenzenesulfonyl chloride as the raw material and methanol as the solvent, and carrying out a desulfurizing coupling reaction under the action of copper acetate to obtain para-dinitrophenyl sulfone, and then adding sodium hydrosulfite to the reaction system to obtain the dapsone. According to the invention, the methanol is taken as the solvent, and sodium hydrosulfite is added as the reducing agent to obtain the dapsone by the one-pot method under the action of the copper acetate without any ligand, the protection of an inert gases and separation; and the total yield can be 88%.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Dapsone to treat rosascea
The methods described herein provide treatment of rosacea using topical formulations of dapsone. The methods also provide treatment of rosacea with topical dapsone in combination with other active agents, including metronidazole. The methods avoid negative hematologic side effects, including hemolysis and hemolytic anemia, that are associated with oral administration of dapsone.
Owner:ALLERGAN INC
Kit for detecting HLA-B*1301 gene
ActiveCN103173538AMicrobiological testing/measurementFluorescence/phosphorescenceLeprosyReference genes
The invention discloses a kit for detecting HLA-B*1301 gene. The kit comprises the following components: 2*PCR Mix, a target gene detection mix, a reference genes detection mix, a positive reference group pMED-B*1301 and deionized water, wherein the gene detection mix comprises 5'-ggtctcacatcatccagagg-3', and a downstream primer 5'ttcctctgcgacgtcgcg-3'; and the reference genes detection mix comprises an upstream primer beta-F:5'- ctgggacgacatggagaaaa-3', and a downstream primer beta-R:5'- aaggaaggctggaagagtgc-3'. As HLA-B*1301 has close relationship with DHS and has the potential to be the biomarker for predicting dapsone allergic syndrome morbidity of Chinese people, by researching and developing the kit to detect the prediction marker HLA-B*1301 of the dapsone allergic syndrome morbidity, the dapsone allergic syndrome morbidity of leprosy patients can be monitored, therefore, the kit has important clinical significance.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
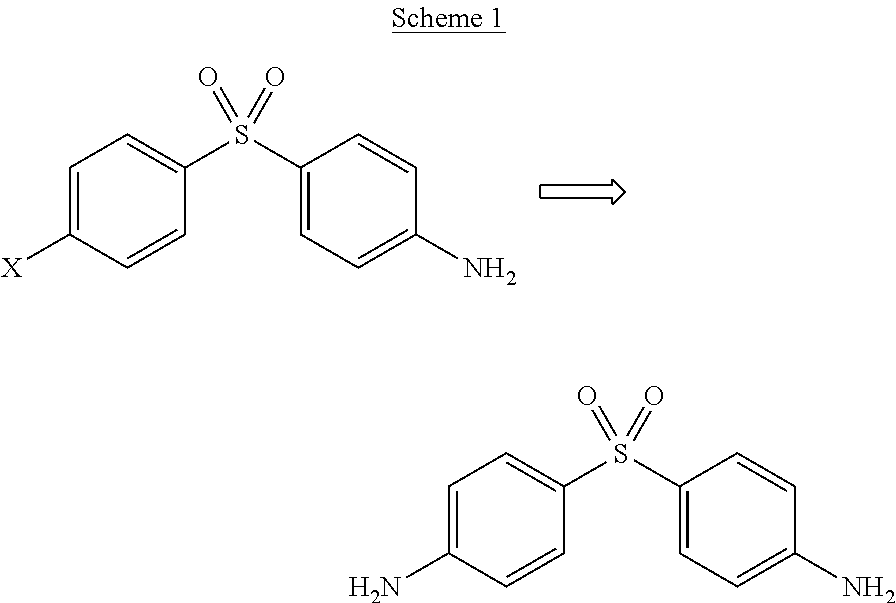
Process for the synthesis of Dapsone and its intermediates
ActiveUS9845289B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDapsonePhotochemistry
Owner:SEEGPHARM
Dapsone-containing gel preparation and preparation method thereof
InactiveCN106265489AQuality improvementGood curative effectOrganic active ingredientsAerosol deliveryGel preparationLeprosy
The invention provides a dapsone-containing gel preparation and a preparation method thereof and belongs to the new technical field of medicines. The effective ingredient of the gel preparation is dapsone which is a sulfone type bacteriostatic agent, and the gel preparation has a sterilization effect when used in a large-dosage manner, has been used for treating leprosy clinically previously and is found that the gel preparation can also be used for treating nodular acne. A penetration enhancer is preferably selected for the gel preparation, so that the gel preparation has a good penetration property and a high deep sterilization function, can clear deep acne, is convenient to use, attractive in appearance and easy to clean and has no irritation to skin.
Owner:BEIJING VENTUREPHARM BIOTECH
Emulsive composition containing dapsone
InactiveUS20130018104A1Stabilises emulsive compositionAvoid separationAntibacterial agentsOrganic active ingredientsActive agentPerylene derivatives
The present invention relates to a topical, emulsive composition containing Dapsone or its derivative. The inventive composition incorporates emollients and Dapsone or its derivative in a stable emulsion. The stability is achieved through the use of a combination of certain surfactant mixtures and an enhancer providing solubility of the Dapsone.
Owner:ALLERGAN INC
Methods of increasing the efficacy of peroxides
InactiveUS7323185B2Good curative effectCosmetic preparationsHeavy metal active ingredientsDERMATOLOGY/SKINBenzoyl peroxide
This invention relates to methods of increasing the efficacy of peroxides such as benzoyl peroxide in the treatment of skin conditions such as acne. In a preferred embodiment, the invention relates to methods of increasing radicals formed by peroxides on / in the skin, more specifically near / in the comedone, for topical use in dermatology. In a specific embodiment, the invention relates to the use of transitional metals such as Cu(1) and ferrous ions to increase the efficacy of peroxides such as benzoyl peroxide. In another embodiment, the invention relates to a method by which a peroxide such as benzoyl peroxide and its activator are added to the skin surface at the same time. In another embodiment, the invention relates to the use of a more soluble form of peroxide such as benzoyl peroxide to increase its efficacy. In another embodiment, the invention relates to the addition of a side chain to a peroxide such as benzoyl peroxide so that it is activated by light. In a further embodiment, the invention relates to the addition of a tertiary amine to a peroxide such as benzoyl peroxide at the time of skin application, to improve the efficacy of the peroxide. In another embodiment, the invention relates to the addition of dapsone or other material to a peroxide such as benzoyl peroxide to improve its efficacy.
Owner:BURKHART CRAIG N
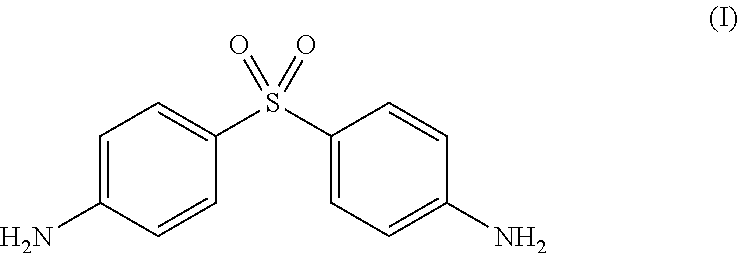
Application of dapsone in protection of integrity of blood brain barrier
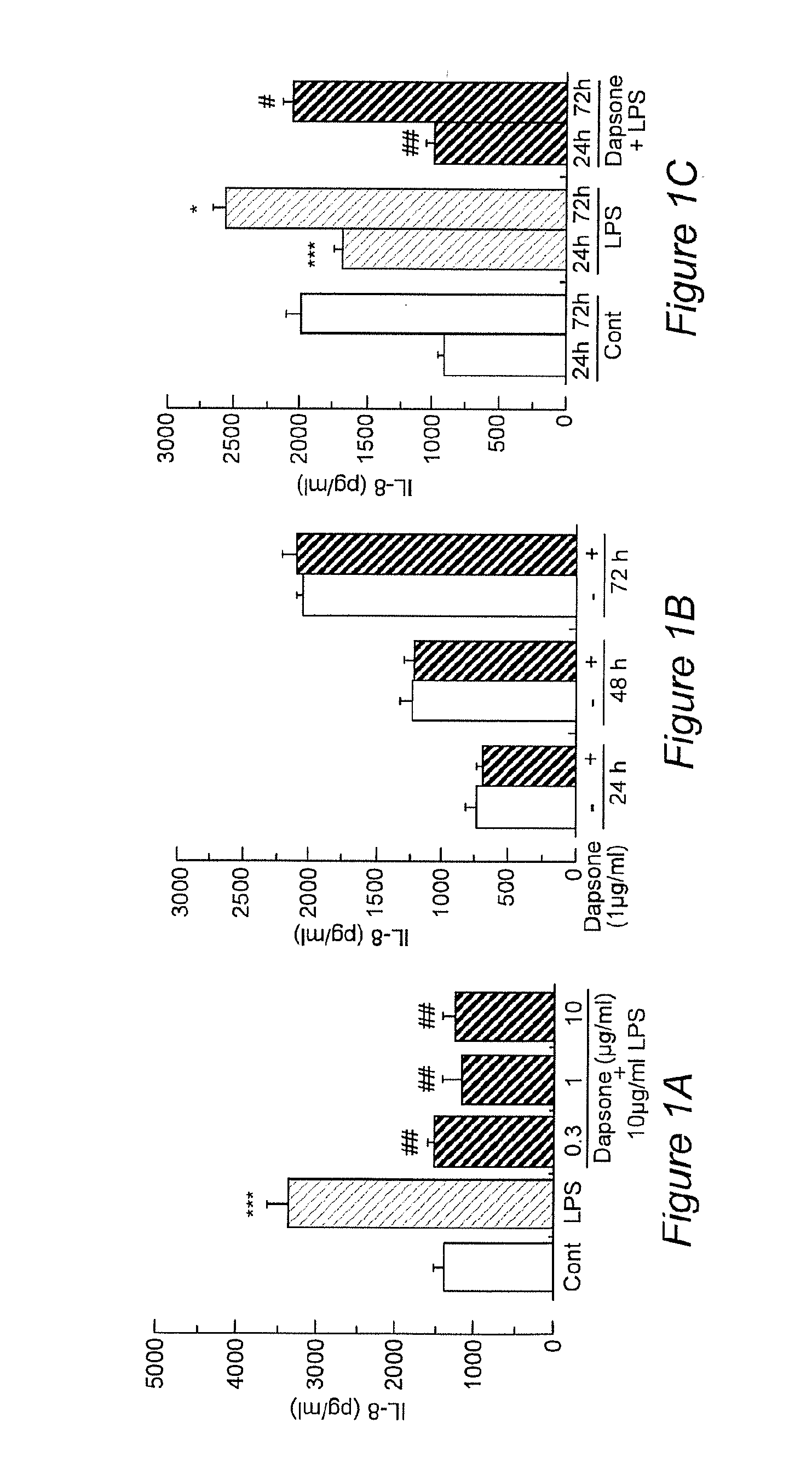
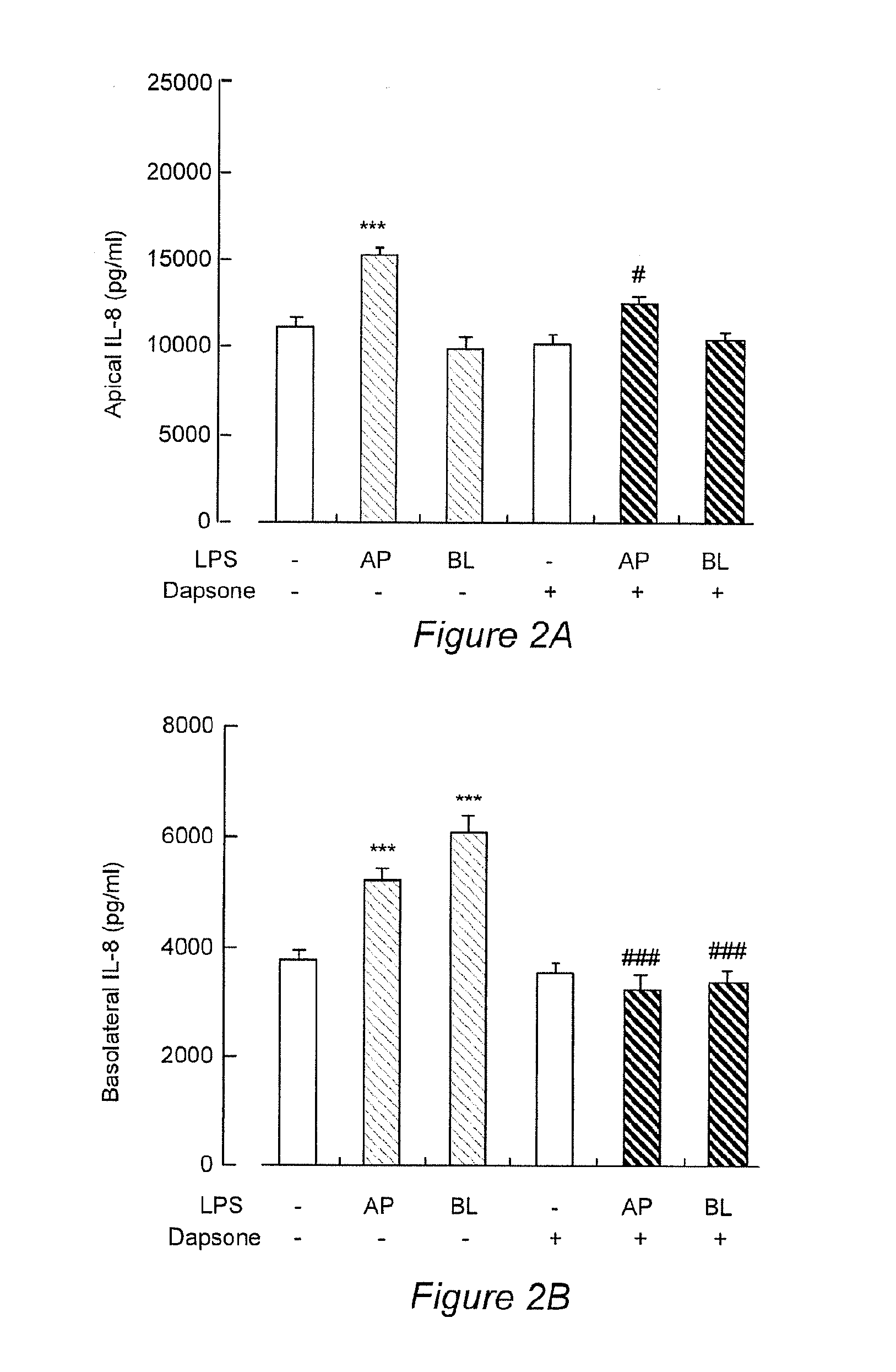
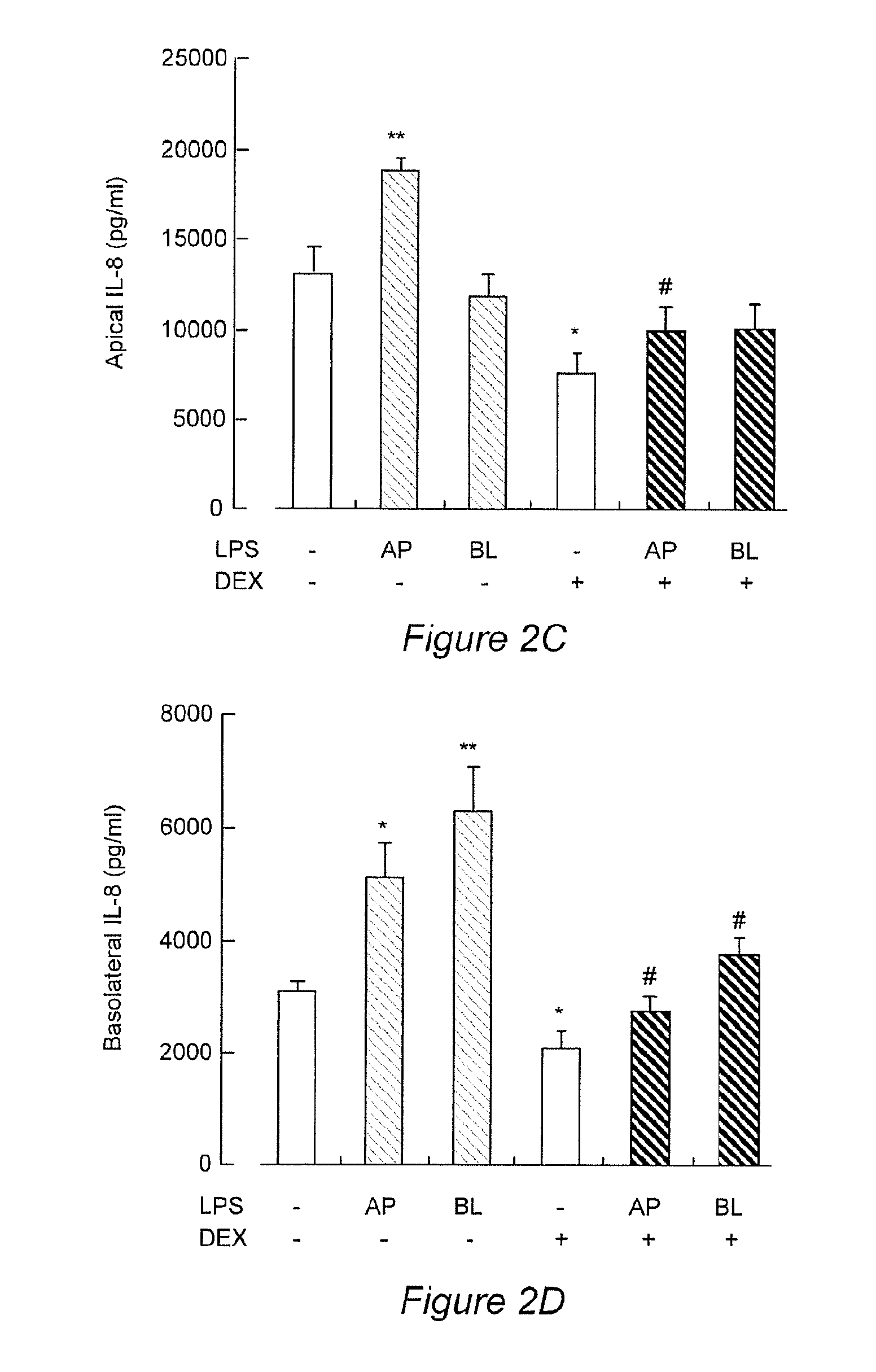
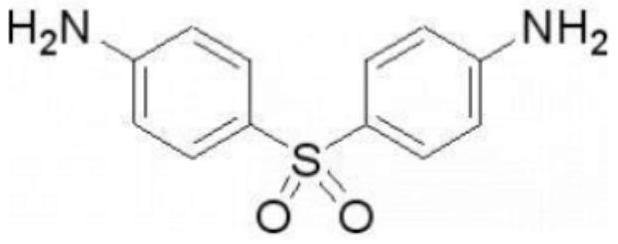
InactiveCN104069091AImprove integrityOrganic active ingredientsNervous disorderInflammatory factorsDapsone
The invention relates to an application of dapsone (a chemical name is 4,4'-diamino diphenyl sulfone) in protection of integrity of blood brain barrier. The application of a model of blood brain barrier damage caused by lipopolysaccharide inflammatory factors shows that dapsone can be used for reducing the ROS level of a blood vessel of a mouse brain by reducing the activity of DADPH oxidase and the level of DADPH oxidase 2 so as to increase expression of tight junction protein and finally relieving blood brain barrier leakage caused by LPS. The application of the dapsone shows that dapsone can be used for protecting the integrity of the blood brain barrier. The structure preparation of dapsone is as shown in the specification.
Owner:崔德华
Synthesis method for dapsone
The invention belongs to the field of medical chemistry, and discloses a preparation method for dapsone, wherein the method comprises the following steps: with chlorobenzene as a raw material and sulfuric acid as a solvent, carrying out a dehydration reaction to obtain dichlorodiphenyl sulfone, and then adding an ammoniation reagent into the reaction system, to prepare dapsone. The dilute sulphuric acid is used as the solvent, no any ligand, no protection with inert gas or no separation is required, sodium hydrosulfite is added as a reducing agent, and dapsone can be prepared. The method can obviously improve the yield and quality of dapsone.
Owner:BEIJING VENTUREPHARM BIOTECH
Aerosolized Dapsone as a Therapy for Inflammation of the Airway and Abnormal Mucociliary Transport
InactiveUS20150040894A1Increased risk of infectionRise in antibiotic resistant bacterial strainsBiocidePowder deliveryDiseaseObstructive Pulmonary Diseases
Aerosolized dapsone (or alternatively, an aqueous formulation of dapsone) is used to treat airway inflammation, particularly chronic neutrophil-dominated inflammation. Diseases that may be prevented or treated by the methods include chronic obstructive pulmonary diseases (COPDs), asthma, cystic fibrosis, and others.
Owner:VIRGINIA COMMONWEALTH UNIV
Dapsone compound suspension as well as preparation method and application thereof
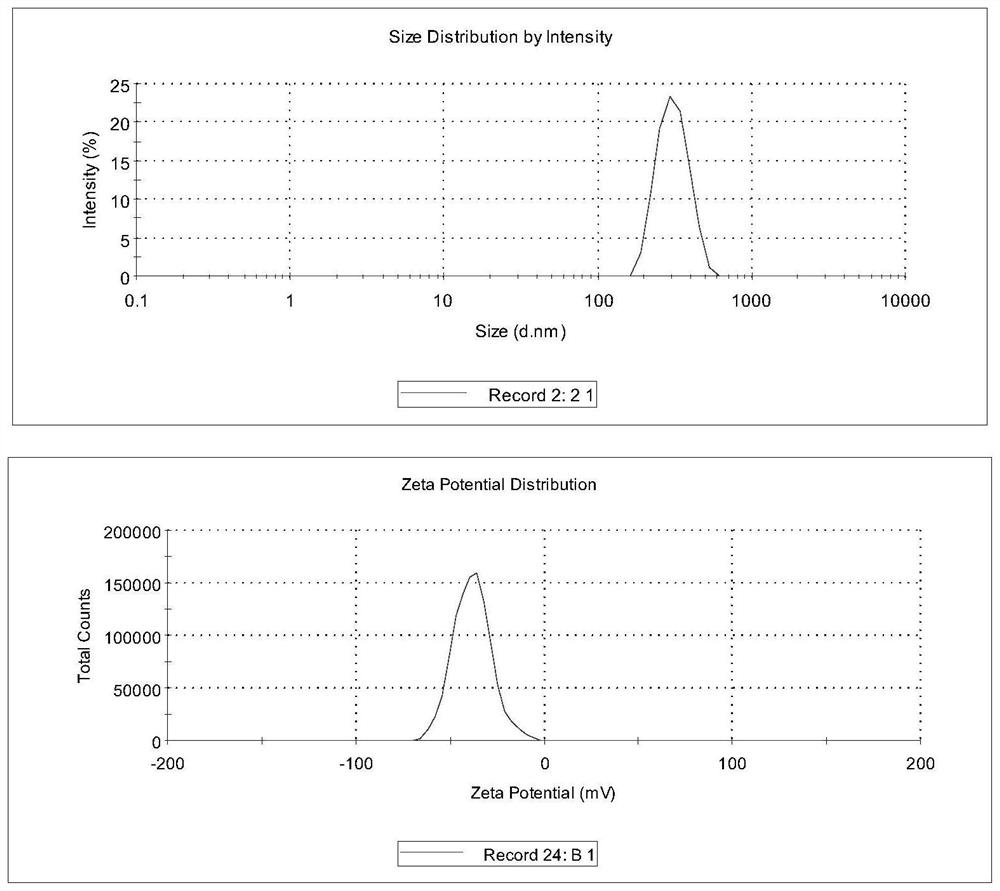
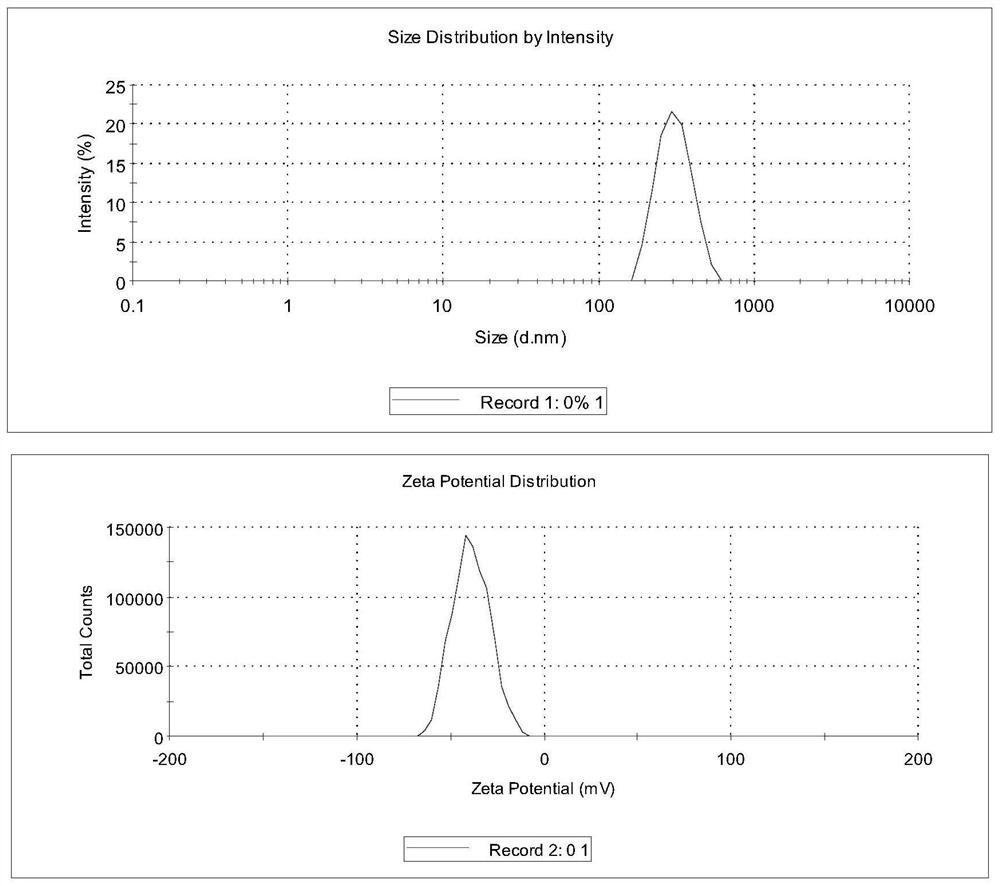
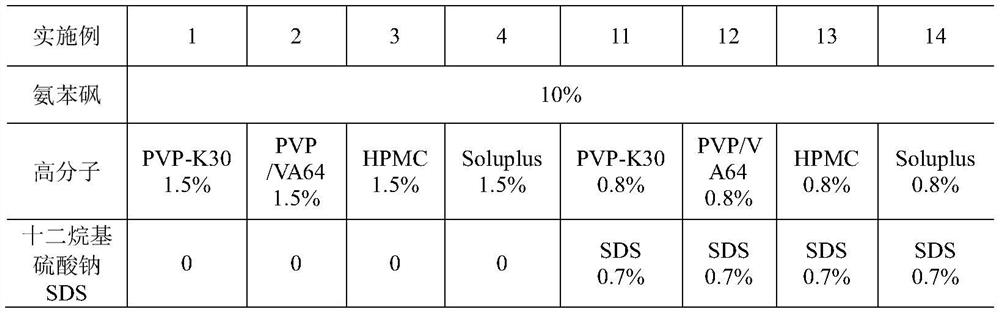
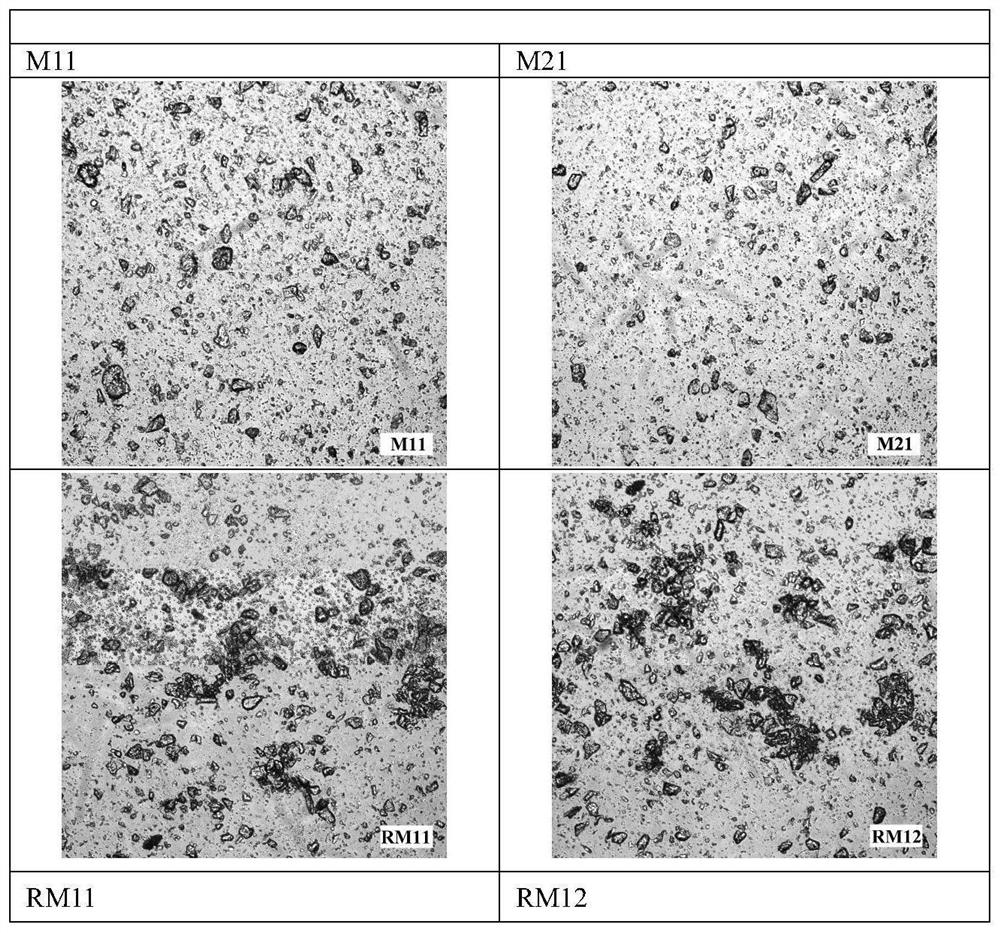
ActiveCN113577022AEffective control of key quality attributesLarge particle sizeAntibacterial agentsOrganic active ingredientsPolyethylene glycolPyrrolidinones
The invention discloses a dapsone compound suspension as well as a preparation method and application thereof, the suspension comprises a dapsone compound and a polymer, and the polymer is selected from one or more of the following components: polyvinylpyrrolidone, hydroxypropyl methyl cellulose, hydroxyethyl cellulose, PVP / VA, Soluplus and polyethylene glycol. Compared with the prior art, the suspension disclosed by the invention has the advantages of large drug loading capacity, narrow particle size distribution, good stability, easiness in production and preparation, industrial applicability and the like.
Owner:NANJING XINTONG RUIYI MEDICINE SCI & TECH
Transdermal drug delivery composition containing dapsone compound and preparation method of transdermal drug delivery composition
PendingCN113577013AConvenient treatmentImprove transdermal deliveryAntibacterial agentsAerosol deliveryCyclodextrinTopical preparation
The invention discloses a transdermal drug delivery composition containing dapsone compounds and a preparation method of the transdermal drug delivery composition. The composition is mainly prepared from the following raw materials: dapsone compounds and cyclodextrin. The preparation method comprises the following steps: micronizing the dapsone compound, then fully mixing and dispersing the micronized dapsone compound and cyclodextrin in a water-soluble medium to prepare a solution, a micron suspension or a nano suspension, and finally mixing the solution, the micron suspension or the nano suspension with pharmaceutically acceptable auxiliary materials and / or matrixes to obtain the medicine. The external preparation of the composition disclosed by the invention has the characteristics of remarkable skin targeted delivery effect, improved preparation stability, reduced skin irritation and good industrialization feasibility of the preparation process, and shows better application potential.
Owner:NANJING XINTONG RUIYI MEDICINE SCI & TECH
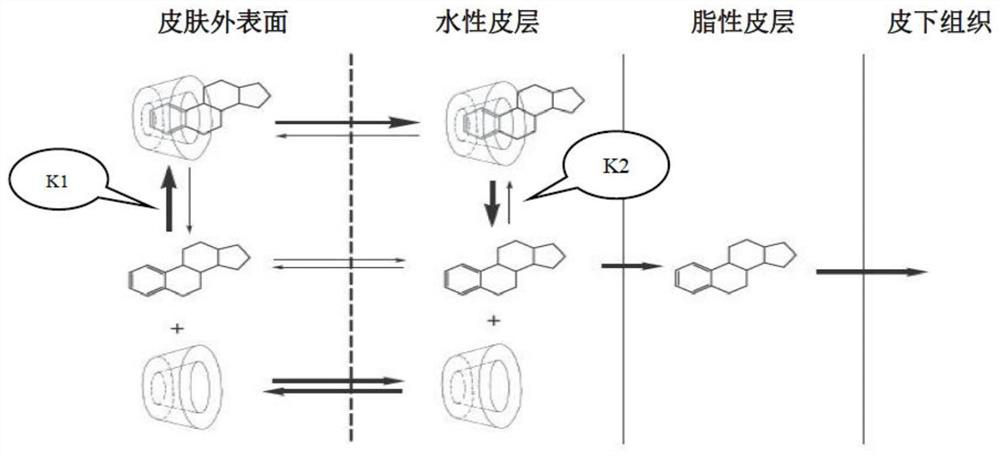
Topical treatment with dapsone in g6pd-deficient patients
The present invention provides a pharmaceutical carrier system comprising a dermatological composition that is a semi-solid aqueous gel, wherein dapsone is dissolved in the gel such that the dapsone has the capacity to cross the stratum corneum layer of the epidermis, and wherein the composition also contains dapsone in a microparticulate state that does not readily cross the stratum corneum of the epidermis. The present invention also provides methods of treating dermatological conditions in G6PD-deficient patients with the composition, while avoiding adverse hematologic effects.
Owner:ALLERGAN INC
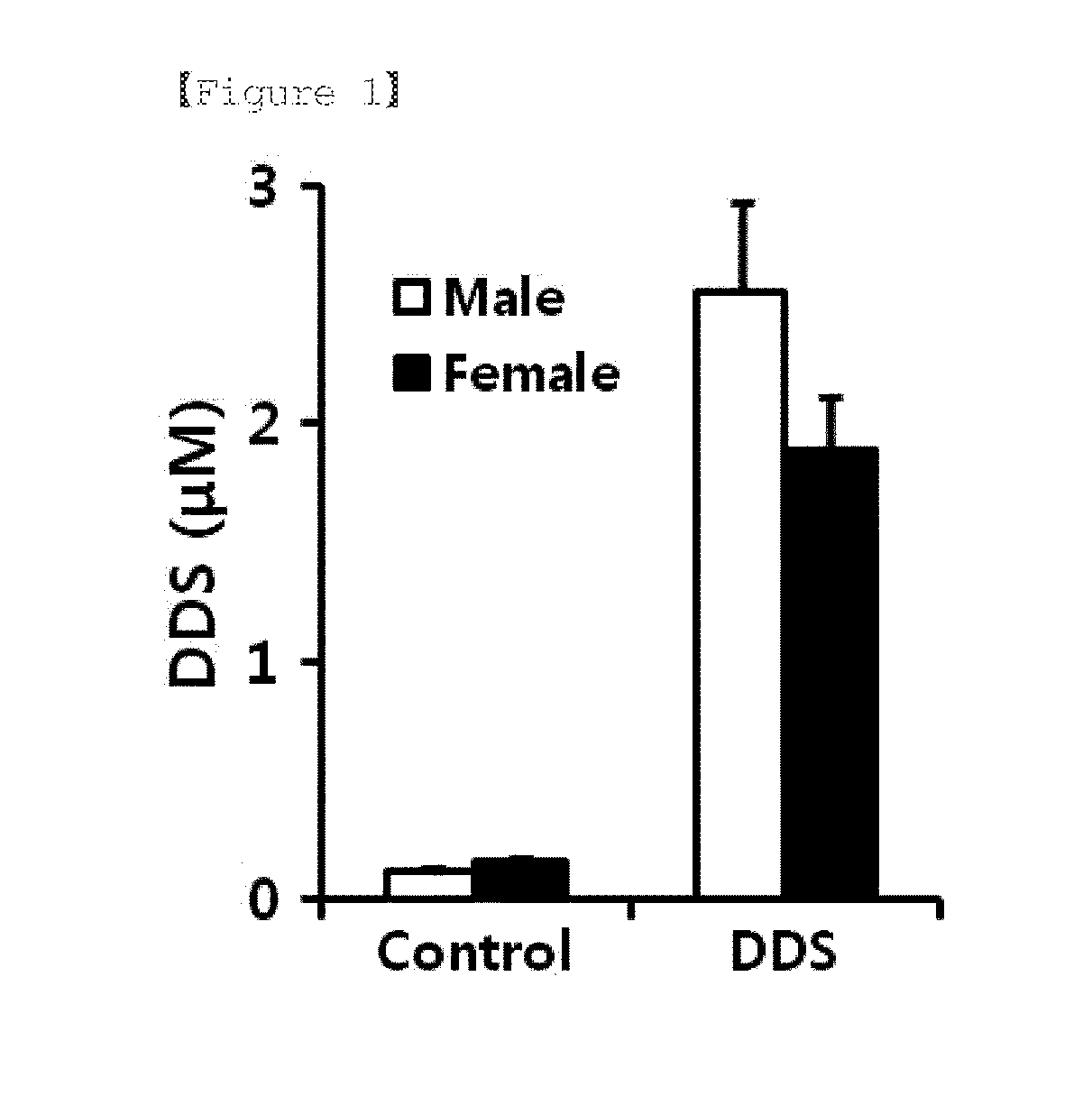
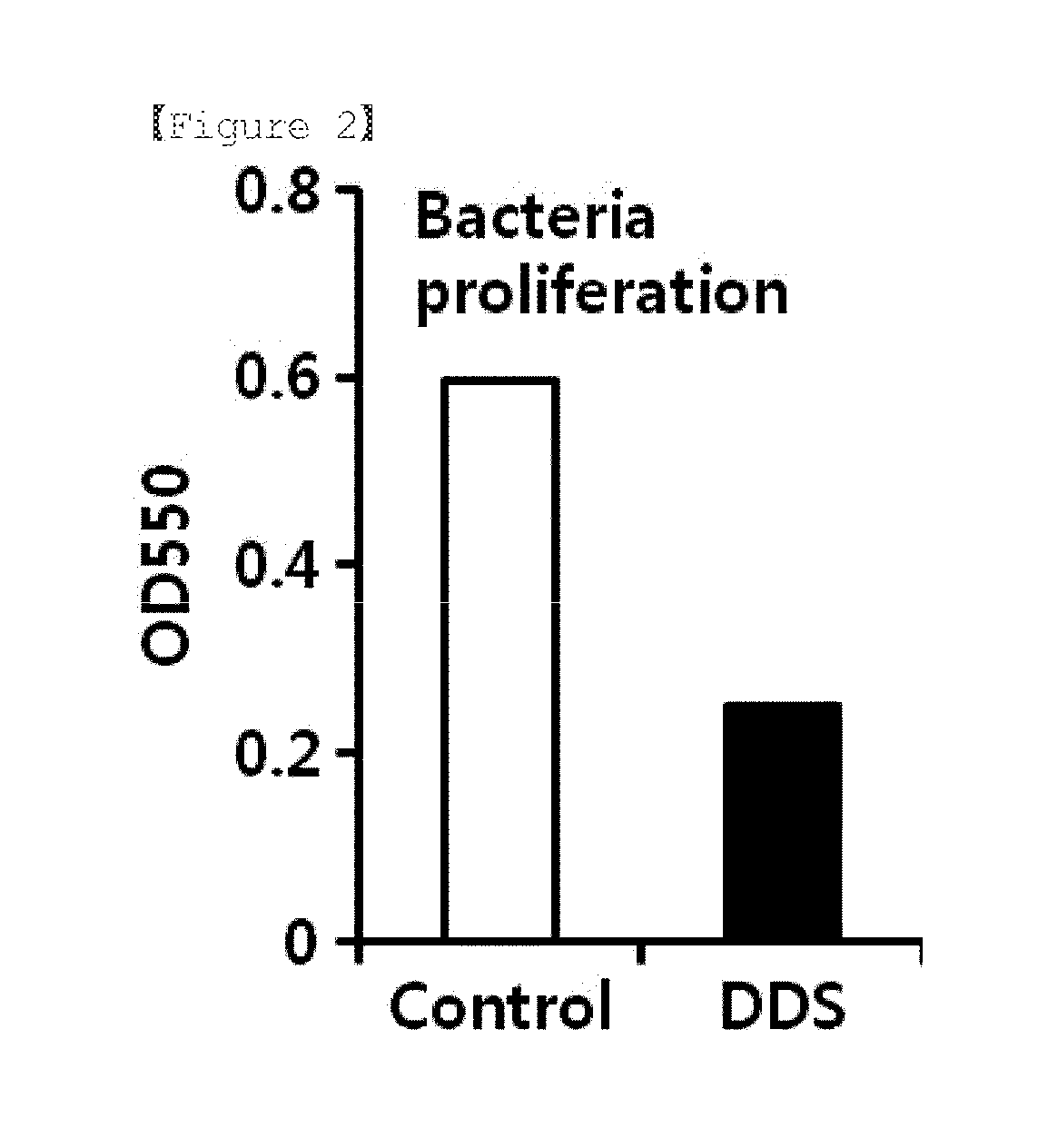
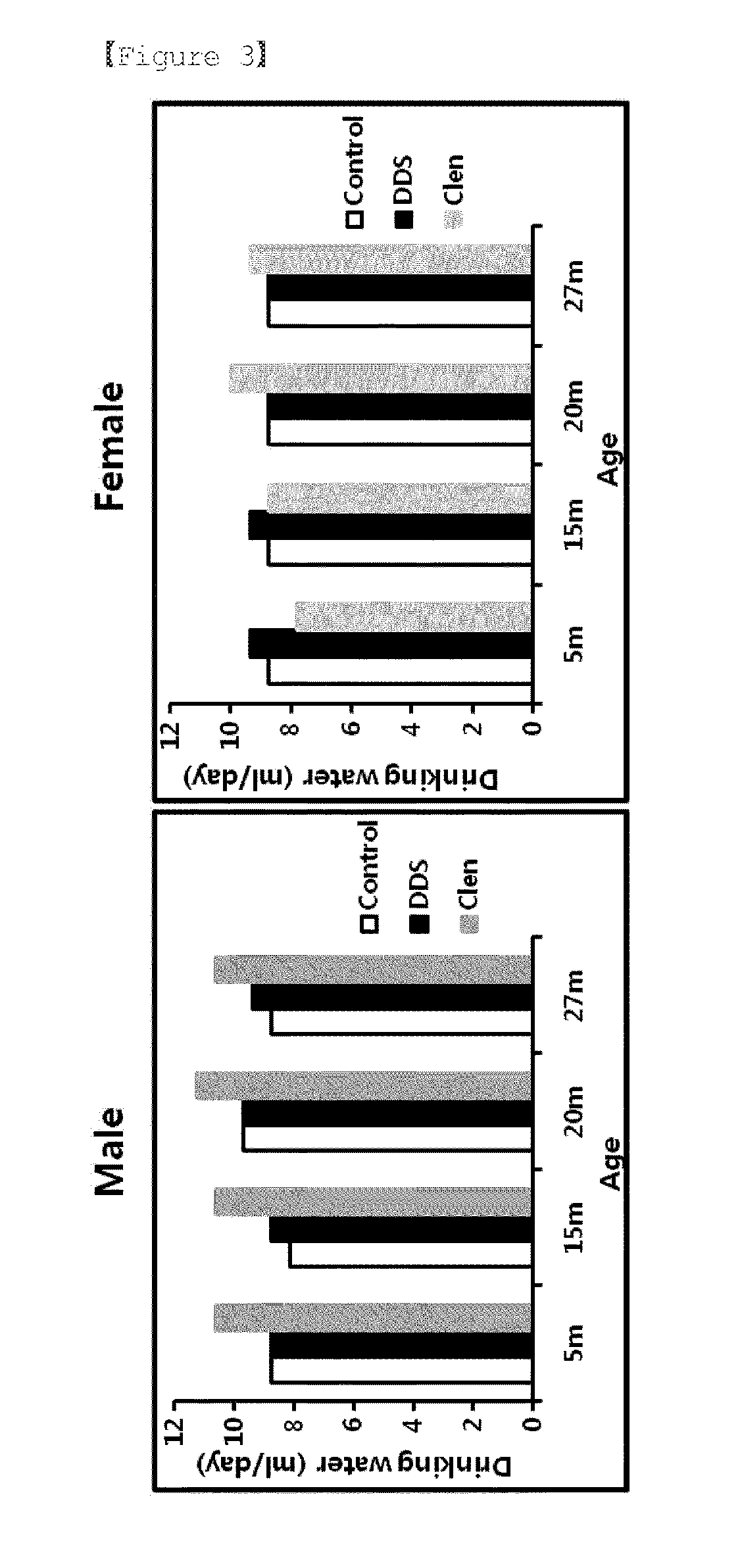
Pharmaceutical Composition for Preventing or Treating Muscle Wasting-Related Disease Comprising Diaminodiphenylsulfone or Pharmaceutically Acceptable Salt Thereof
ActiveUS20150031773A1Increase muscle massEffective recoveryBiocideOrganic compound preparationMuscle lossMuscle functions
The present invention relates to a pharmaceutical composition for preventing or treating a muscle wasting-related disease comprising diaminodiphenylsulfone (Dapsone; DDS) or a pharmaceutically acceptable salt thereof as an active ingredient. The composition according to the present invention may be effectively used to prevent or treat a muscle wasting-related disease by increasing muscle mass, preventing muscle loss and effectively restoring muscle function.
Owner:SAMSUNG ELECTRONICS CO LTD
Use of dapsone as a neuroprotector in cerebral infarction
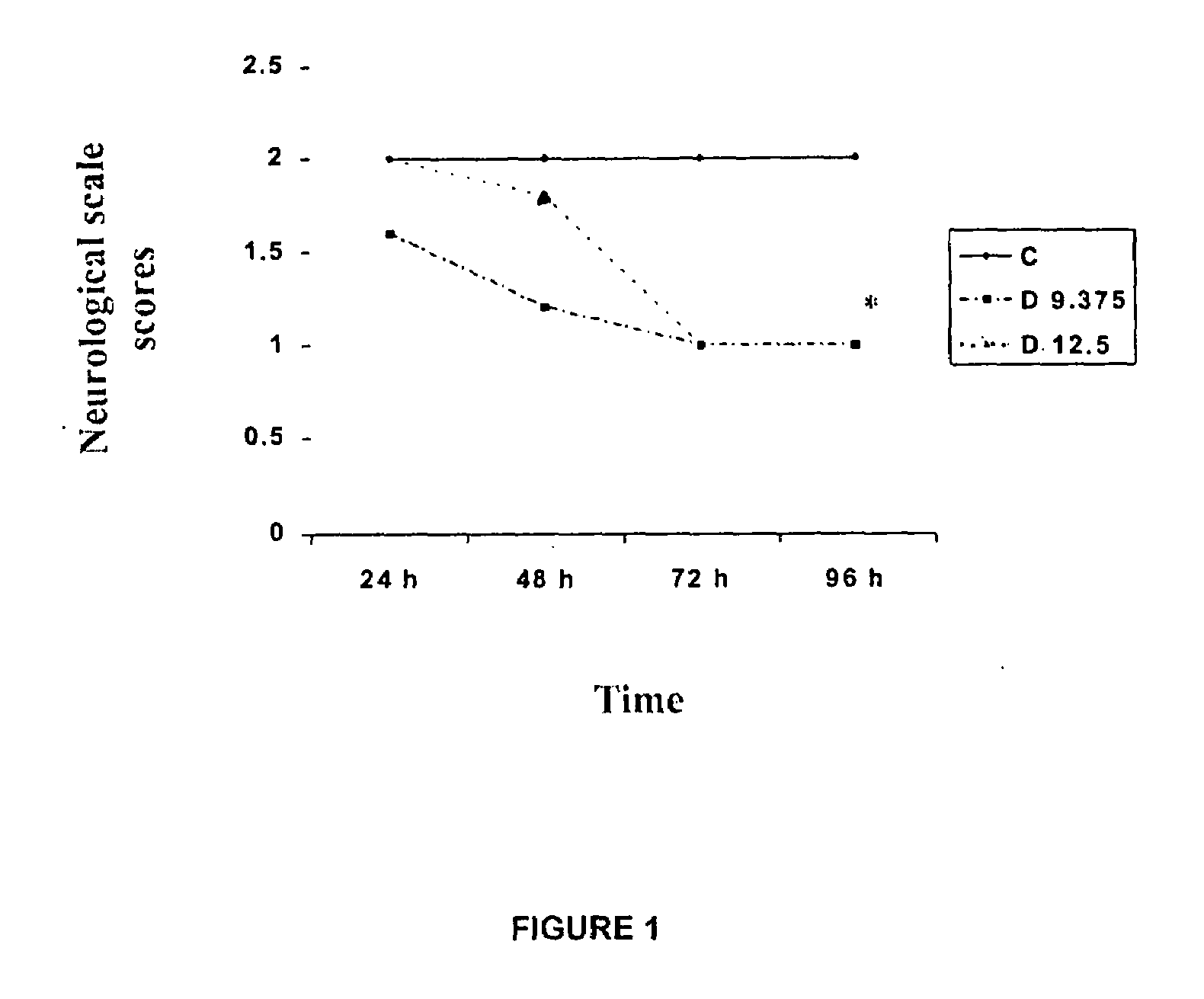
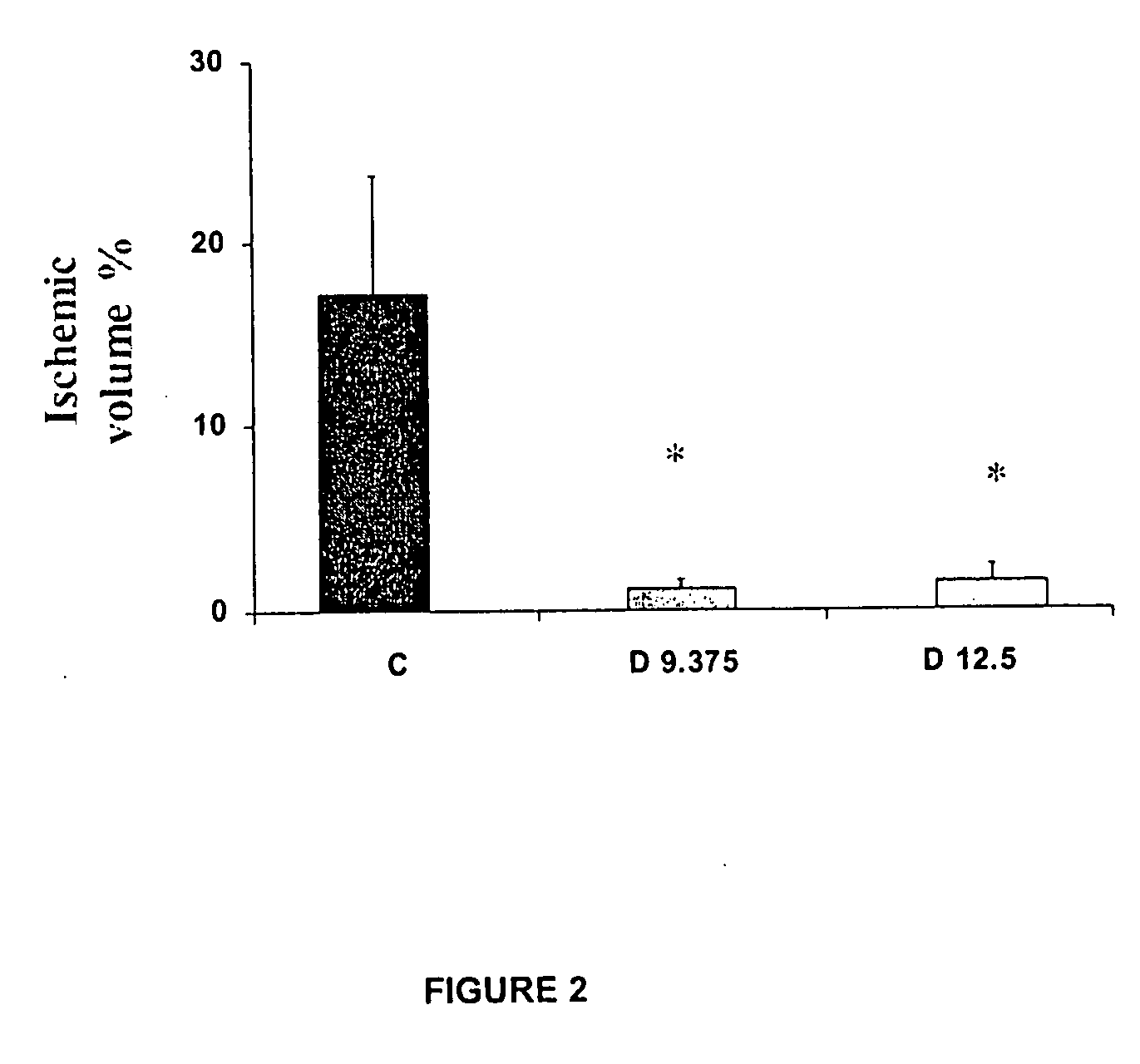
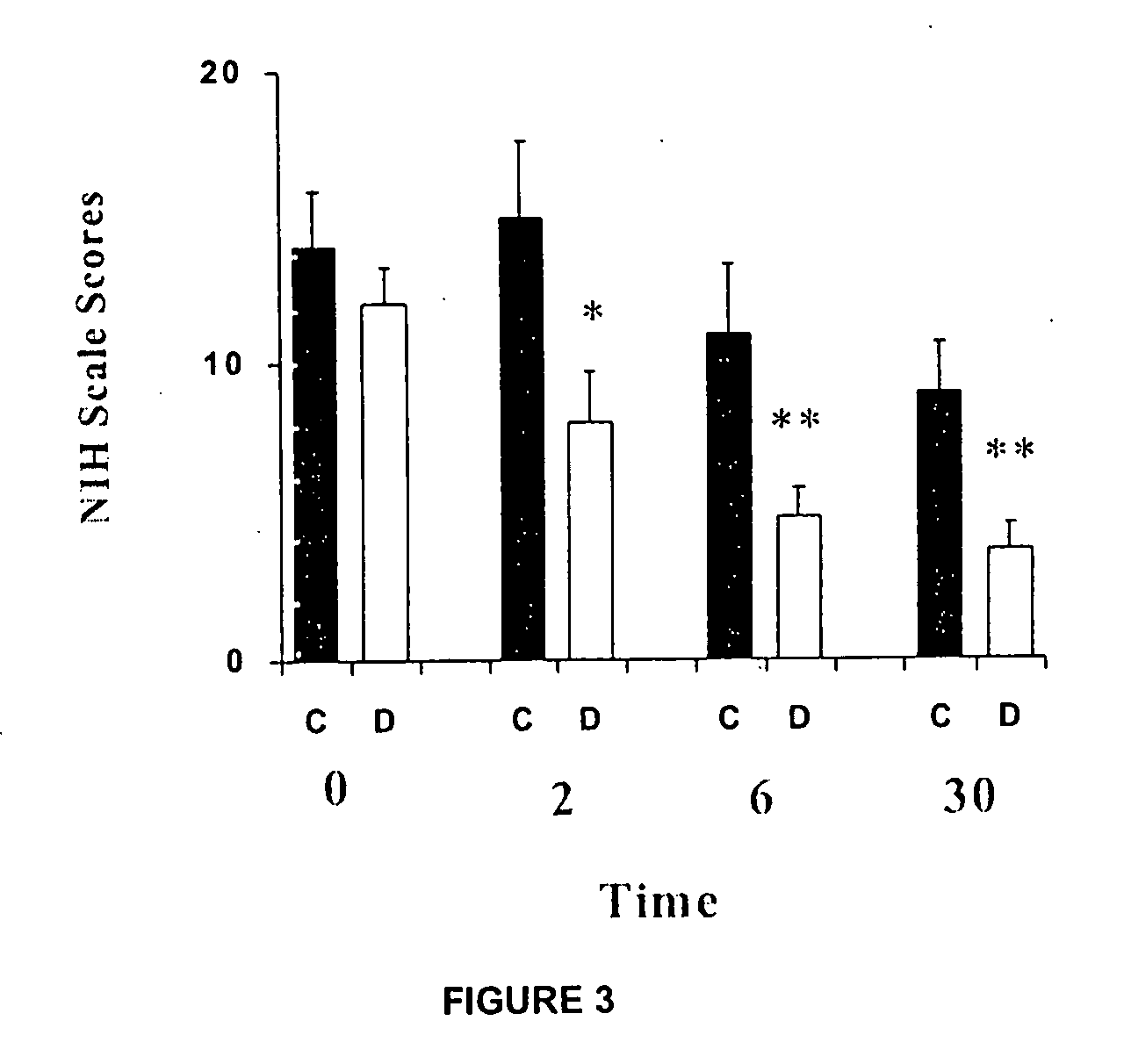
InactiveUS20100063159A1Organic active ingredientsBiocideAcute cerebral infarctionCentral sulcus artery
The use of dapsone is the first effective treatment against the disabling consequences associated with cerebral infarction in patients. Dapsone was evaluated as a neuroprotector in the cerebral infarction model produced by the occlusion of the middle cerebral artery in rats and in patients suffering from acute cerebral infarction caused by thromboembolism. In both studies, dapsone displayed a reduction of between 70 and 90% in the adverse effects which occur as a consequence of the infarction.
Owner:UNIV AUTONOMA METROPOLITANA +1
Protectant for UV-induced skin damage
The present invention provides a method for protecting against UV radiation-induced skin damage. Specifically, compositions including dapsone are administered to provide UV protection. The dapsone compositions may be administered orally, or by other parenteral routes, such as topically, transdermally, by inhalation, and the like.
Owner:ALLERGAN INC
Combination of dapsone with other Anti-acne agents
InactiveUS20110305747A1Good curative effectBiocideCosmetic preparationsEffective treatmentBULK ACTIVE INGREDIENT
A composition suitable for topical application that contains at least two active ingredients, one of these being dapsone and one selected from the group consisting of adapalene, tazarotene and treinion for the effective treatment of acne and other dermatological conditions.
Owner:ALLERGAN INC
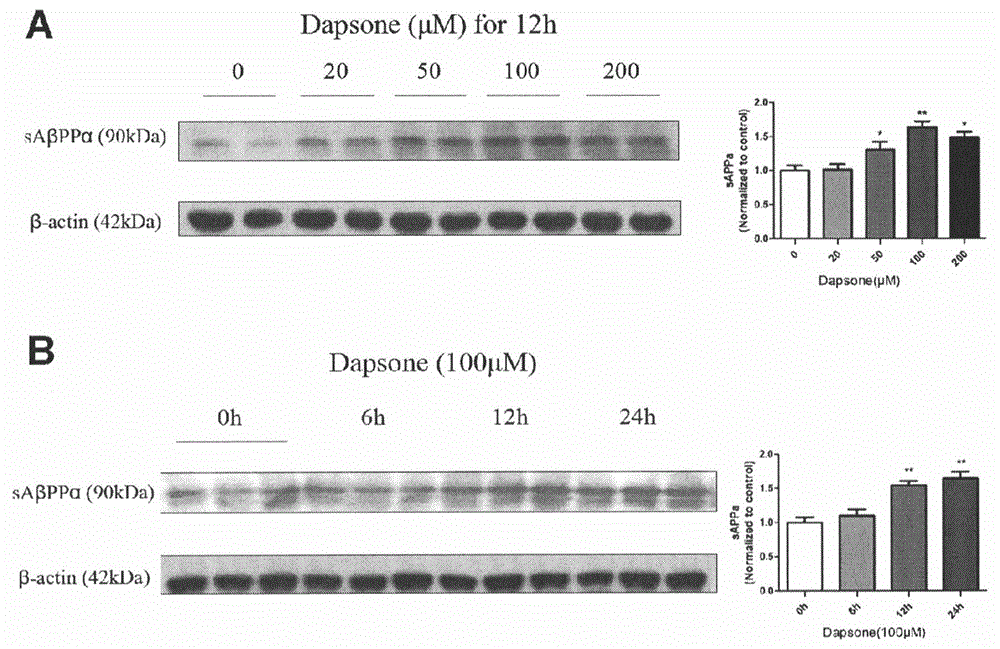
Application of dapsone to preparation of alpha-secretase enhancer
InactiveCN103976989ANo toxicityImprove the level ofOrganic active ingredientsNervous disorderMice brainPharmaceutical medicine
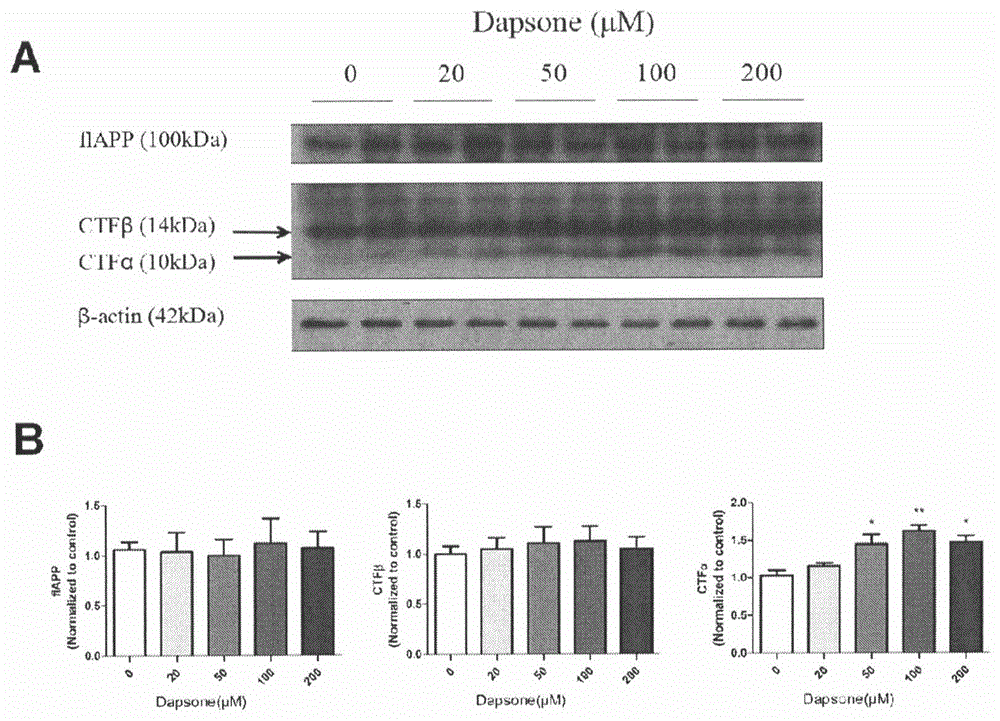
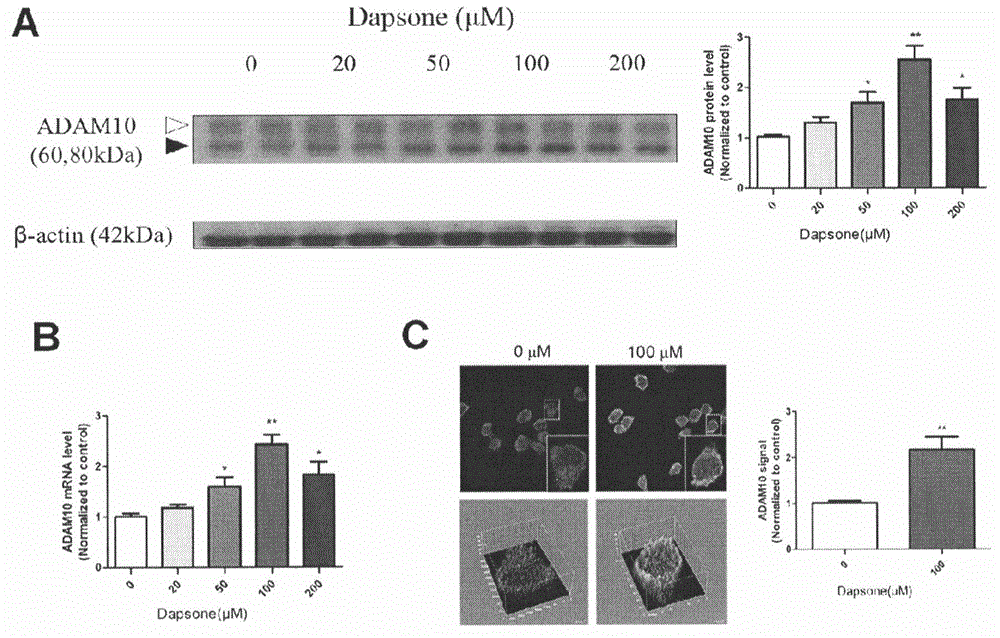
The invention relates to an application of dapsone (Chemical name: 4,4'-diamino diphenyl sulfone) to preparation of an alpha-secretase enhancer. By adopting dapsone, the level of alpha-secretase ADAM10 (A-Disintegrin And Metalloprotease 10) of N2a-APP cells is raised, so that the level of soluble APPalpha (sAPPalpha) is raised. By using the compound provided by the invention, the density of intracerebral synapse of a mouse can be increased, and the cognizing function is improved. Moreover, the compound provided by the invention can be combined with pharmaceutically-acceptable medicinal auxiliary materials or carriers, and can be applied orally or through rectum. The structural formula is shown in the specifications.
Owner:崔德华
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com