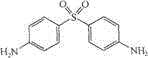Dapsone-containing gel preparation and preparation method thereof
A dapsone and gel technology, which is applied in the field of preparation of dapsone gel preparations, can solve the problems of low absorption in vitro, reduce patient compliance, affect the curative effect of dapsone gel, and the like, achieve good curative effect, increase Skin permeability, the effect of killing deep fungus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] This embodiment is in the form of a gel. (Based on 1000g)
[0023]
[0024] Preparation Process:
[0025] a) Preparation of liquid medicine: Add Tween 80 (60g) and the pulverized active ingredient dapsone (50g) in the prescription into water (200g), heat in a water bath at 45°C to dissolve;
[0026] b) Preparation of matrix: weigh ethyl p-hydroxybenzoate (3g) and add it to ethanol (20g), heat it in a water bath at 45°C to dissolve it, then add glycerin (30g), propylene glycol (10g) and the rest of the water (565g), then slowly add hydroxypropylmethylcellulose (62g) to the above solution under stirring, stir well, make swelling and disperse evenly, and prepare a gel matrix;
[0027] c) Add the liquid medicine dropwise into the gel matrix, stir evenly, and divide into packages.
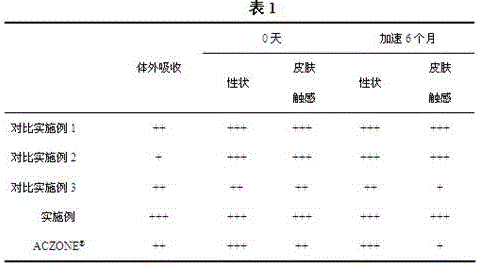
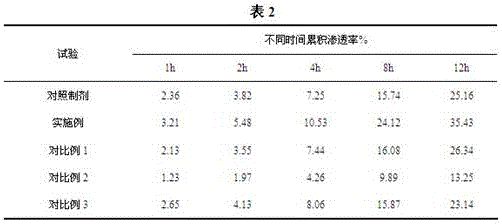
[0028] In order to highlight the advantages and feasibility of this process, we will use several comparative examples and examples for parallel comparison.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com