Dapsone compound suspension as well as preparation method and application thereof
A technology of suspension and dapsone, which is applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problem that the drug with transdermal rate cannot reach the effective drug therapeutic dose, release rate and transdermal rate change, and skin delivery target. Troubles such as tropism instability, to achieve the effect of reducing the drug penetration rate, reducing the skin retention, and maintaining a stable drug particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] One, the preparation research of embodiment
[0060] Nanosuspension and Gel Prescription Form
[0061]
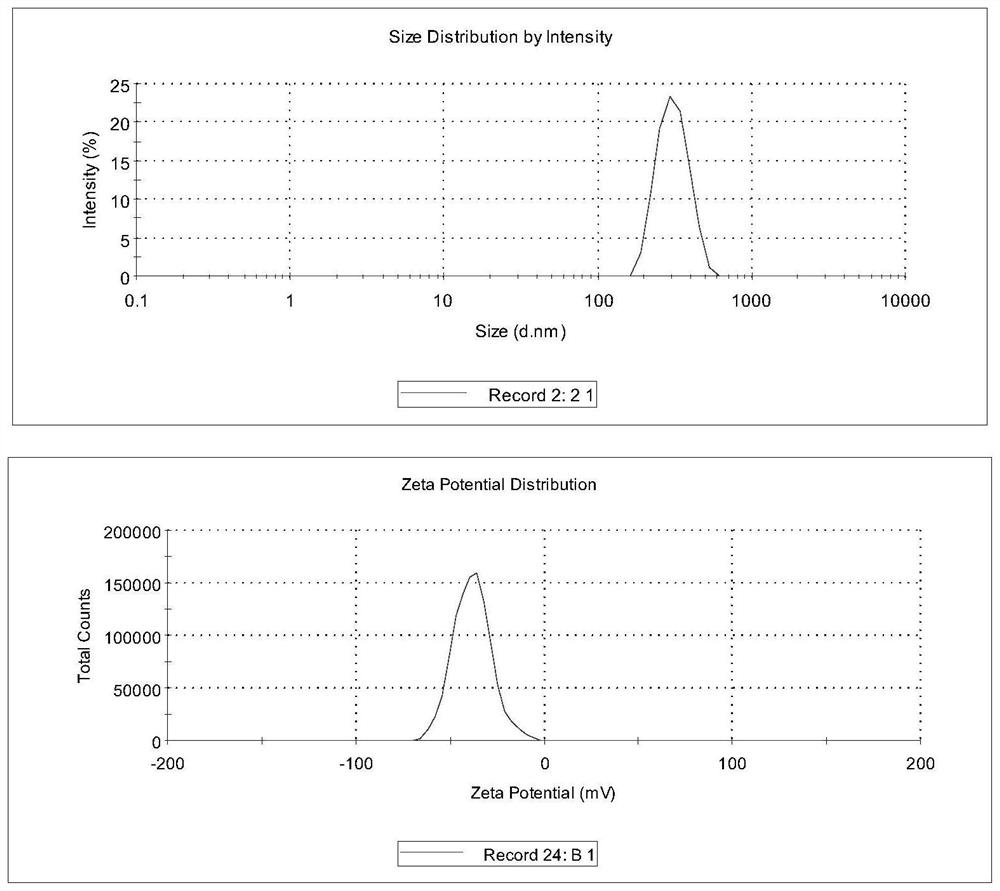
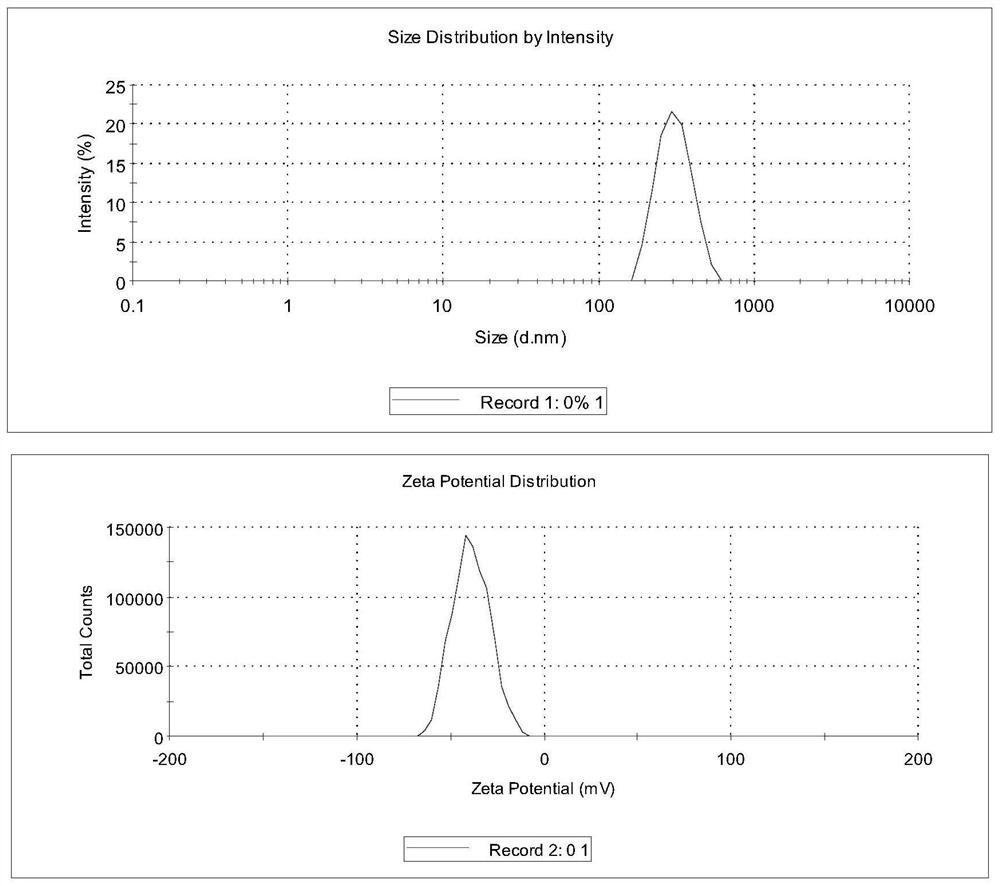
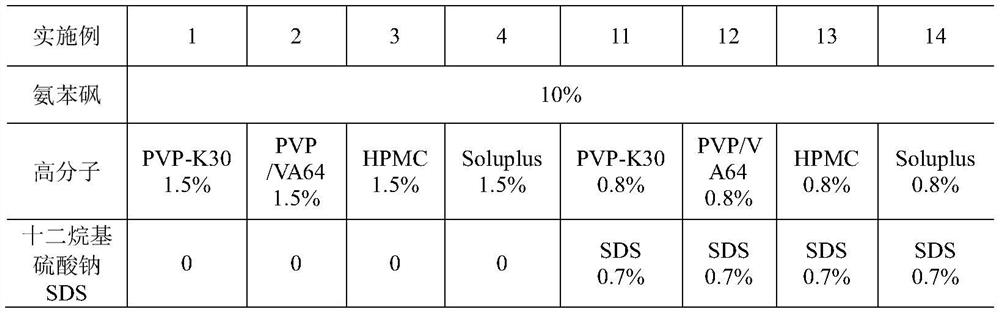
[0062] Examples 1-4 prepared drug nanosuspension by wet grinding method. The suspension prescription contains 10% dapsone and 1.5% polymer. Dapsone is crushed through a 150-mesh sieve, dispersed in 10 times the amount of pure water to prepare the initial mixture, and put into the inner cavity of a wet circulation grinder. The mixture is mixed with equal volumes of zirconia grinding beads (Ф0.5 mm), and ground at 3000 rpm for 40 minutes, and the average particle diameter of dapsone in the obtained suspension is 300-400 nm.
Embodiment 5
[0063]Example 5 is similar to Example 1, but the suspension formulation contains 10% dapsone, 1.6% PVP-K30, 0.6% HPMC-K4M, and wet grinding for 50 minutes. The average particle size of dapsone in the resulting suspension was 296.3 nm. The film-spraying agent is prepared by adding appropriate amount of auxiliary materials into the suspension. Preparation prescription: 5% dapsone, 2% PVP-K30, 0.3% HPMC-K4M, 5% HPβCD, 30% ethanol, 3% glycerin, 0.05% ethylparaben, 0.1% disodium edetate , make up pure water to 100%. Preparation process: Dissolve the remaining auxiliary materials in ethanol or pure water according to the prescription of the preparation, add the drug suspension while stirring successively, make up the pure water to 100%, and stir thoroughly at 100-200r / min to obtain the product. The dapsone content in the obtained spray film agent is 4.98%, and the average drug particle diameter is 246.3nm.
Embodiment 6
[0064] Example 6 is similar to Example 1, but the suspension formulation contains 15% dapsone, 2% PVP-K30, and 1% PEG4000. Dapsone was crushed through a 150-mesh sieve, dispersed in 7 times the amount of pure water to prepare an initial mixture, and ground with zirconia beads (Ф0.5 mm) at 3500 rpm for 40 minutes to obtain a drug nanosuspension. The dapsone content in the obtained suspension was 14.95%, and the average drug particle diameter was 393.7nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com