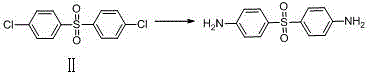Synthesis method for dapsone
A synthetic method, the technology of dapsone, applied in the field of medicinal chemistry, can solve the problems of slow reaction progress, unavailable reagents, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
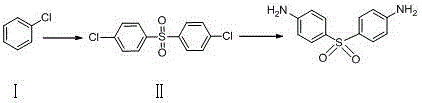
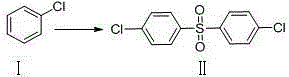
[0015] Example 1: 4,4'-Dichlorodiphenylsulfone
[0016]
[0017] In a 100ml three-neck flask, add 11.26g (0.1mol) of compound I and 20.0ml of concentrated sulfuric acid, react at room temperature for 4 hours, TLC spotting test shows that the reaction is complete, add 20.0ml of pure water to precipitate a solid, filter it with a large amount of The filter cake was washed three times with pure water to obtain Compound II, which was dried overnight in a forced air oven at 40°C to obtain 27.29 g of a light yellow crystalline powder with a yield of 95.02%. MS(+1): 286.96.
Embodiment 2
[0018] Example 2: Dapsone
[0019]
[0020] Measure 50.0ml of ethanol into a 250ml three-neck flask, then slowly add 10.0g (40.27mmol) of 4,4'-dichlorodiphenyl sulfone, 4.30g (80.54mmol) of ammonium chloride solid, stir while adding, and then add 50.0ml of ethanol, heated and refluxed for 5 hours, TLC spotting test showed that the reaction was complete, 30ml of distilled water slowly quenched the reaction, stirred and lowered the temperature, waited for a large amount of solids to precipitate, filtered with suction, washed the filter cake with a small amount of ethanol three times, and blasted at 40 ℃ After drying, 7.9 g of white crystalline powder was obtained, yield: 91.9%. MS (+1): 249.30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com