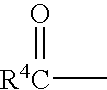Patents
Literature
36 results about "Lopinavir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
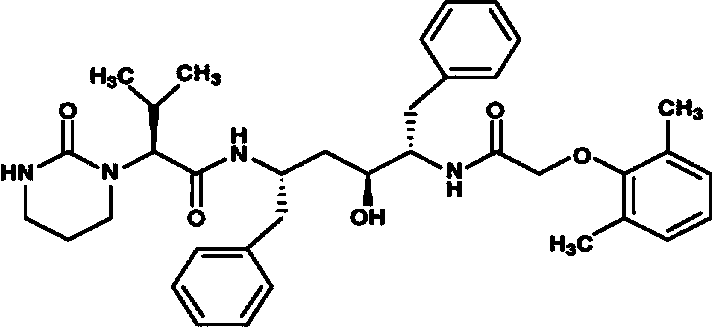
Lopinavir is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir (lopinavir/ritonavir). It was patented in 1995 and approved for medical use in 2000.
Therapeutic compositions and methods
InactiveUS20090093482A1Prolonged systemic exposureIncrease exposureBiocideTripeptide ingredientsMedicineCarboxylic acid
The invention includes methods, compositions, and kits useful for treating a viral infection by coadministering 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or a pharmaceutically acceptable salt thereof, with lopinavir or a pharmaceutically acceptable salt thereof.
Owner:GILEAD SCI INC
Lopinavir preparation and purification process
The invention relates to a lopinavir preparation and purification process, and belongs to the field of drug synthesis. The purification process comprises: dissolving a lopinavir crude product in methanol, cooling to a temperature of 0-5 DEG C, adding water firstly, continuously cooling to a temperature of 5-15 DEG C, carrying out first thermal insulation stirring, standing, separating to obtain a supernatant, adding water secondly to the supernatant in a dropwise manner, stirring until the solid is precipitated, adding water thirdly, carrying out second thermal insulation stirring at a temperature of 10-15 DEG C after completing the water adding, and separating to obtain the lopinavir, wherein the concrete step of the third water adding is that the water is added in a dropwise manner in a plurality of batches within an interval time. According to the present invention, in the purification process, a small amount of the methanol and a large amount of the water are adopted as the purification solvent, such that the toxicity is low, and the cost is low; and with the purification process, the lopinavir crude product with the HPLC purity (peak area%) of less than 99.5%, preferably less than 99.1% and containing more than two single impurities with the content of more than 0.1% can be purified into the lopinavir with the single impurity content of less than 0.1% and the HPLC purity of more than 99.5%, wherein the loss during the purification process is less than 10%.
Owner:福建蔚嘉生物医药有限公司
Method used for preparing Lopinavir using one-pot method
InactiveCN108218791ASimple processShorten the production cycleOrganic chemistryOrganic solventReaction system
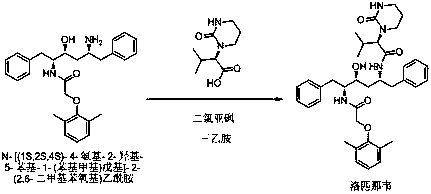
The invention discloses a method used for preparing lopinavir using one-pot method. According to the method, under certain organic solvent conditions, (2S)-(1-Tetrahydropyramid-2-one)-3-methylbutanoicacid is reacted with thionyl chloride so as to obtain (2S)-(1-Tetrahydropyramid-2-one)-3-methyl butyryl chloride; a weak base acid binding agent and N-[(1S,2S,4S)-4-amino-2-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-2-(2,6-dimethylphenoxy)acetamide are added into an obtained reaction system for amidation reaction; after amidation reaction, an obtained product is subjected to post-treatment so as toobtain lopinavir finished product. According to the method, on-pot method is adopted, the process is simple, production period is short, the finial products can be separated and purified easily, synthesis yield is high, the method is economical and is high in feasibility, and is suitable for industrialized production.
Owner:YANCHENG DESANO PHARMA CO LTD
Flavoring systems for pharmaceutical compositions and methods of making such compositions
InactiveUS6911214B2Improve bioavailabilityGreat tasteDispersion deliveryInorganic non-active ingredientsPeppermintsChemistry
A flavoring system for a liquid pharmaceutical composition and pharmaceutical compositions containing such flavoring systems are disclosed. Flavoring systems of the invention include at least one sweetening agent, at least two flavored ingredients, and at least one flavor modifier selected from the group consisting of citric acid, sodium citrate, sodium chloride, and mixtures thereof. At least two of the flavored ingredients are selected from the group consisting of a vanilla flavored ingredient, a peppermint flavored ingredient, a menthol flavored ingredient, a cotton candy flavored ingredient, and mixtures thereof. The one or more sweetening agents comprise glycerin, monoammonium glycyrrhizinate, saccharin sodium, acesulfame potassium, high fructose corn syrup, and / or mixtures thereof. Pharmaceutical compositions of the invention include a flavoring system of the invention, a solvent system, and at least one pharmaceutically active agent, such as lopinavir or derivatives thereof, ritonavir or derivatives thereof, or mixtures thereof. Methods for making such liquid pharmaceutical compositions are also disclosed.
Owner:ABBVIE INC
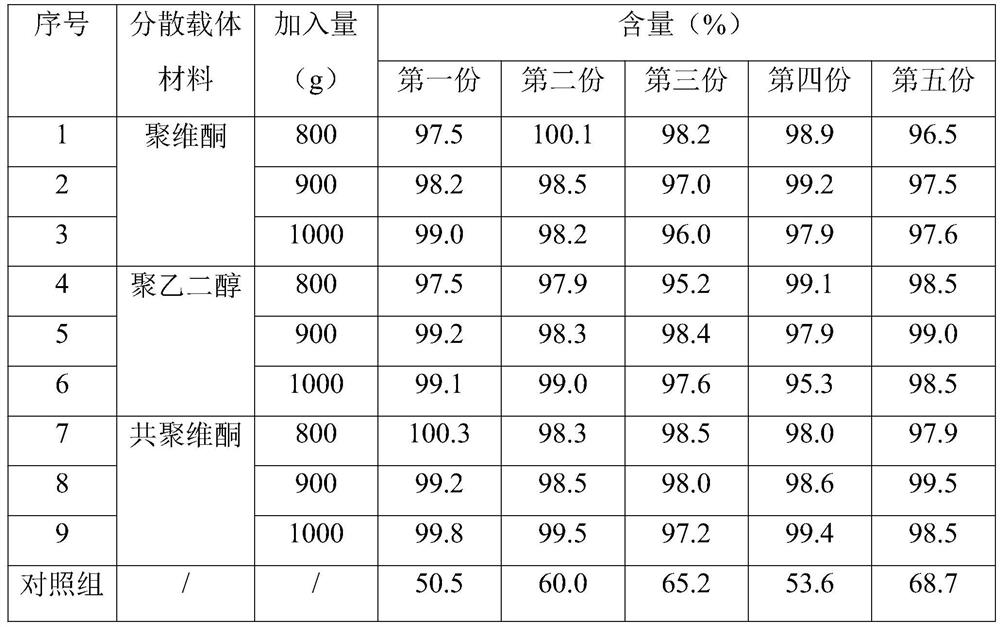
Lopinavir and ritonavir compound high-uniformity nano co-dispersion body and preparation method thereof
ActiveCN103655571ASimple manufacturing methodSuitable for industrial productionOrganic active ingredientsAntiviralsPharmaceutical industryDissolution
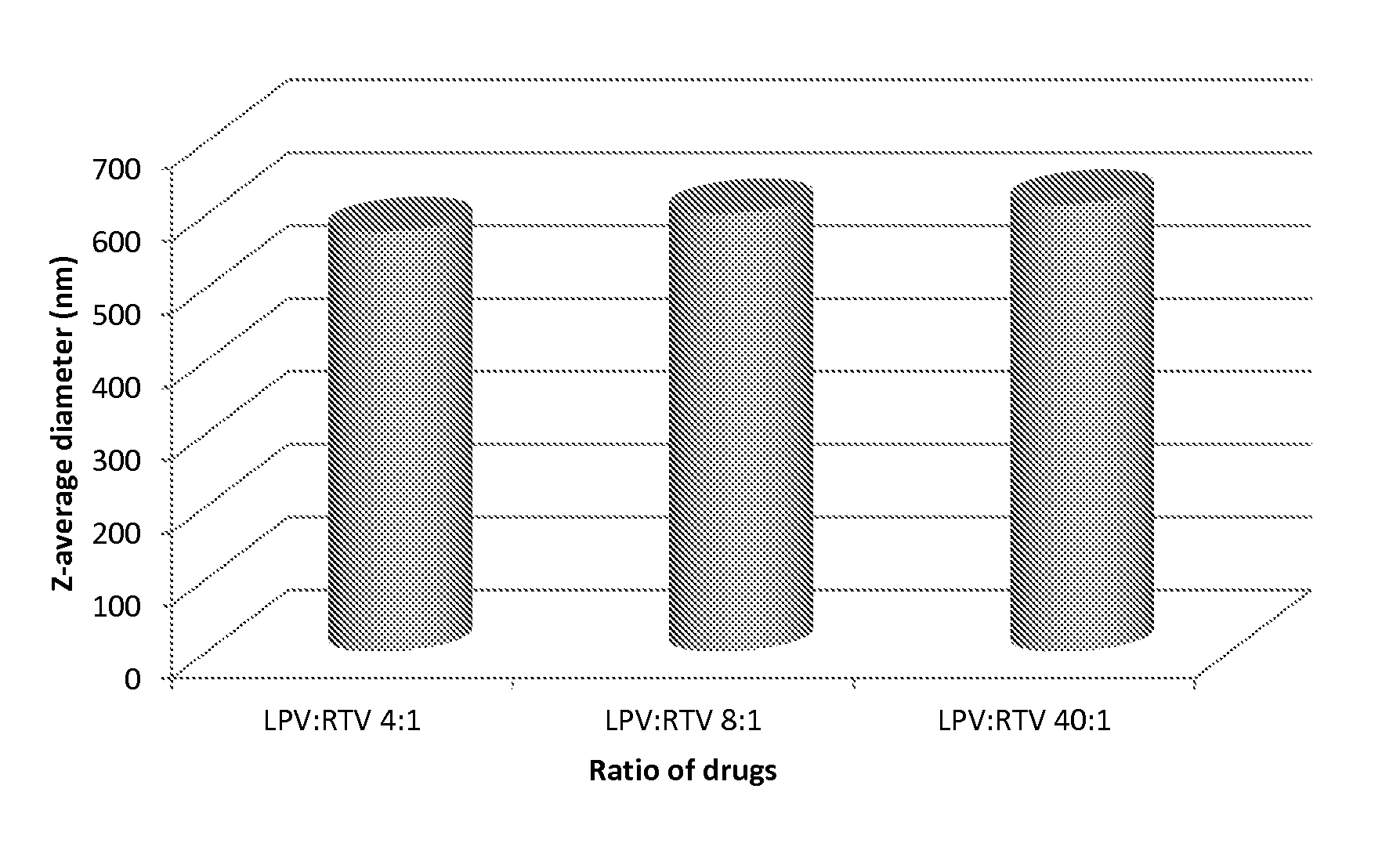
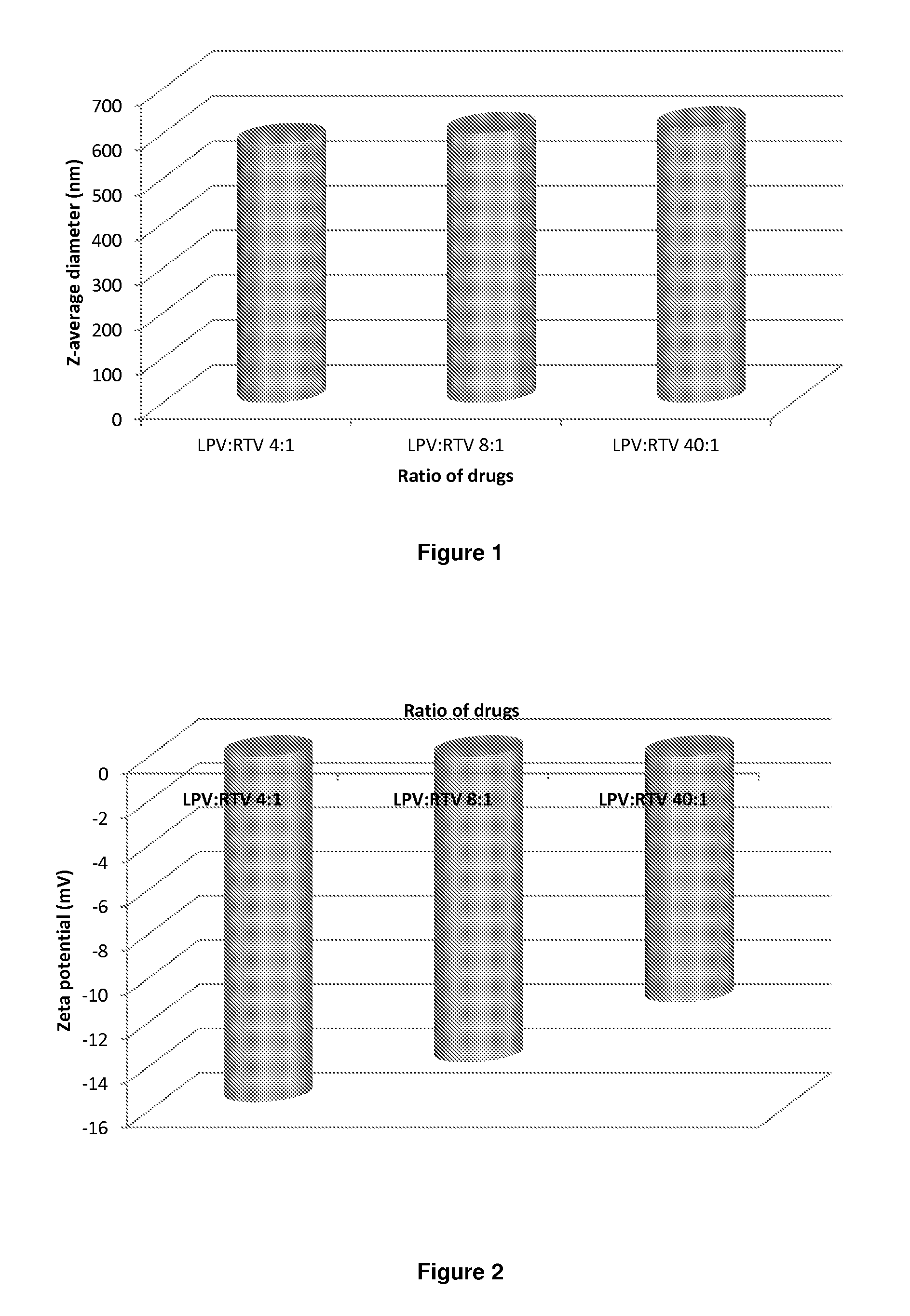
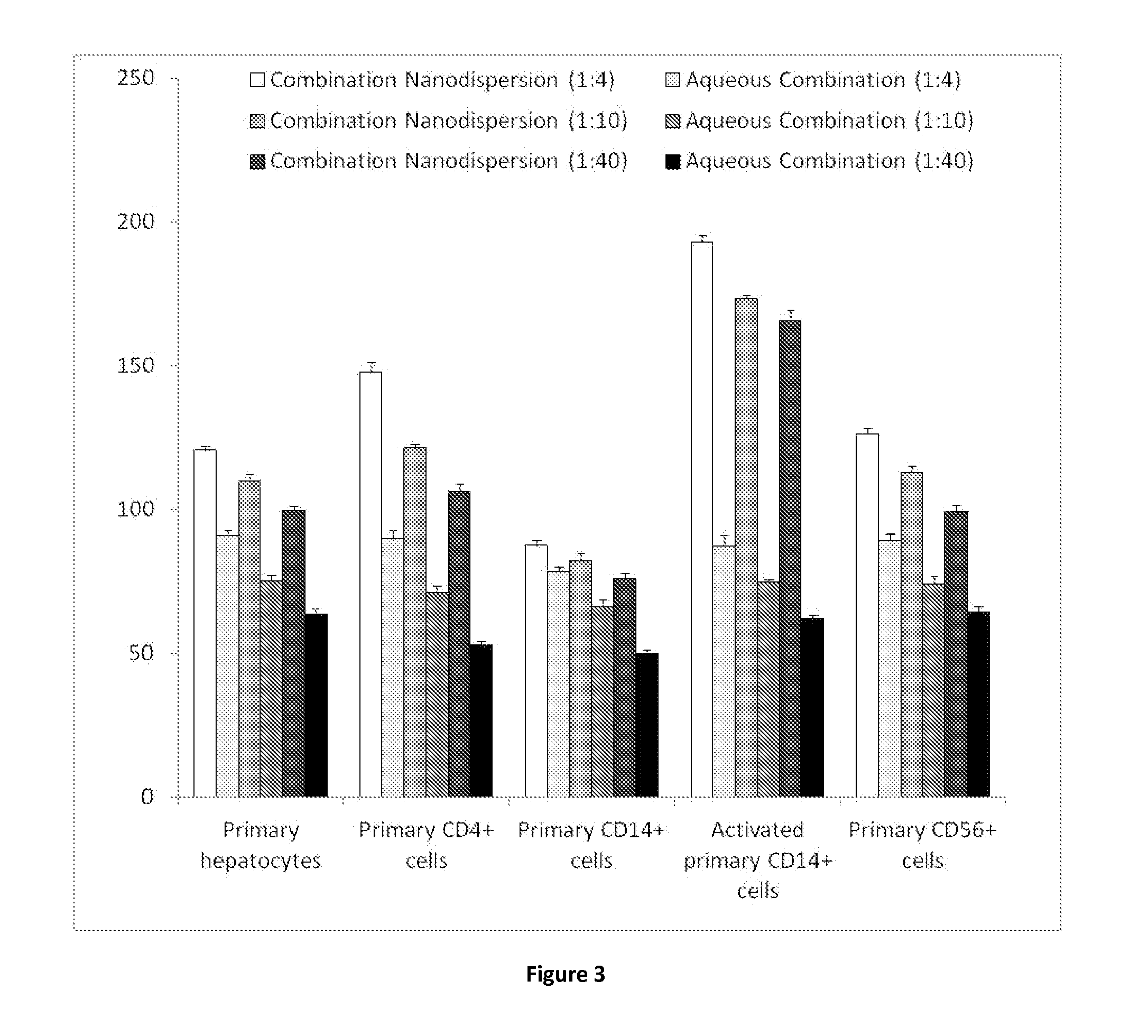
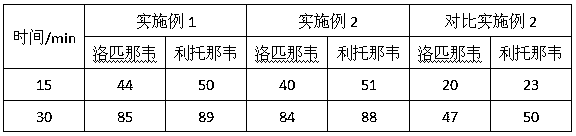
The invention provides a lopinavir and ritonavir compound high-uniformity nano co-dispersion body. The lopinavir and ritonavir compound high-uniformity nano co-dispersion body consists of lopinavir and ritonavir which are taken as active ingredients, a dispersing aid, an anti-agglormeration material, a dispersing stabilizer, a dispersion supporter and a lubricant. The lopinavir and ritonavir which are taken as active ingredients account for 25%-60%. The lopinavir and ritonavir compound high-uniformity nano co-dispersion body prepared by the preparation method disclosed by the invention can be prepared into a single-dose preparation according to a normal method. According to a dissolution rate experiment, the preparation disclosed by the invention has release amount of 40%-80% within 30 minutes. The preparation prepared by the dispersion body disclosed by the invention is high in dissolution rate, and can be used for improving the bioavailability. The lopinavir and ritonavir compound high-uniformity nano co-dispersion body prepared by the preparation method disclosed by the invention satisfies and is superior to mixing uniformity standards. The preparation method disclosed by the invention is simple and easy to implement, can be used for popularizing application of a nano mechanical grinding technology on the pharmaceutical industry and promoting the development of the technology, and is suitable for industrial production and greater in application value.
Owner:SHANGHAI SUNTECH PHARMA +1
Process for the preparation of solid dispersion of lopinavir and ritonavir
An extrusion process for preparation of solid dispersion of lopinavir and ritonavir carried out in twin screw extruder.
Owner:RANBAXY LAB LTD
Lopinavir inhalation aerosol and preparation method thereof
ActiveCN111265499AImprove stabilityAccurate dosePowder deliveryOrganic active ingredientsPulmonary infectionActive agent
The invention belongs to the technical field of medicines, and discloses a lopinavir inhalation aerosol and a preparation method thereof. The lopinavir inhalation aerosol is composed of lopinavir serving as an active ingredient, a propellant, a cosolvent, a surfactant and the like according to a certain ratio . The aerosol inhalant is administrated through the oral cavity and directly acts on thelung to achieve targeted administration. The inhalation preparation disclosed by the invention can target a focus, is accurate in dosage, takes effect rapidly, can quickly improve the pulmonary infection condition, is beneficial to improve the adaptability of an infected person, and avoids gastrointestinal tract absorption so as to reduce side effects on the gastrointestinal tracts.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Flavoring systems for pharmaceutical compositions and methods of making such compositions
Owner:ABBOTT LAB INC
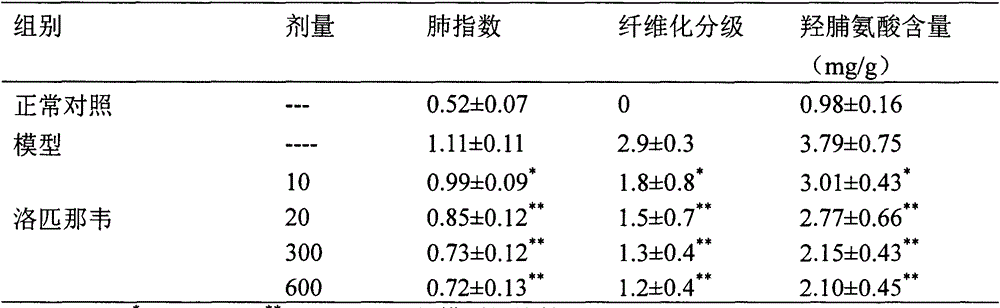
Use of lopinavir in preparation of medicines for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
InactiveCN104083373AOrganic active ingredientsRespiratory disorderApoptosisARDs - Acute respiratory distress syndrome
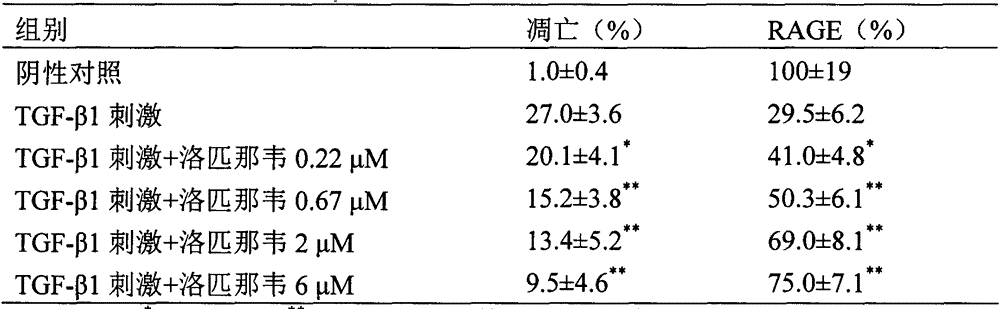
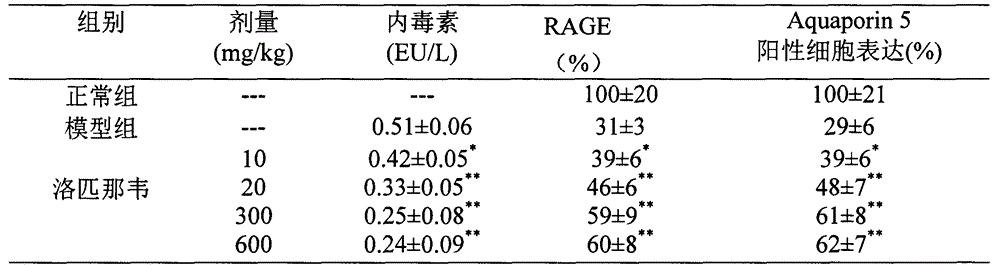
The invention provides a new medical use of lopinavir and specifically relates to a use of the lopinavir in preventing or treating acute lung injury / acute respiratory distress syndrome (ALI / ARDS) and pulmonary fibrosis by inhibiting the apoptosis of type I alveolar epithelial cells, promoting the expression of the receptor for advanced glycation endproducts of the type I alveolar epithelial cells, further inhibiting the occurrence and development of ALI / ARDS and the pulmonary fibrosis and finally preventing or treating ALI / ARDS. In the use, the dosage of the lopinavir ranges from 100mg to 6000mg, preferably from 240mg to 3000mg.
Owner:BINZHOU MEDICAL COLLEGE
Dosage form comprising non-crystalline lopinavir and crystalline ritonavir
InactiveUS20150111909A1Maintain good propertiesGood content uniformityOrganic active ingredientsBiocideActive agentCrystallization
The present invention relates to an oral dosage form comprising non-crystalline lopinavir and crystalline ritonavir. The invention further relates to methods of preparing said oral dosage forms containing the above pharmaceutical active agents.
Owner:RATIOPHARM GMBH
Compositions of lopinavir
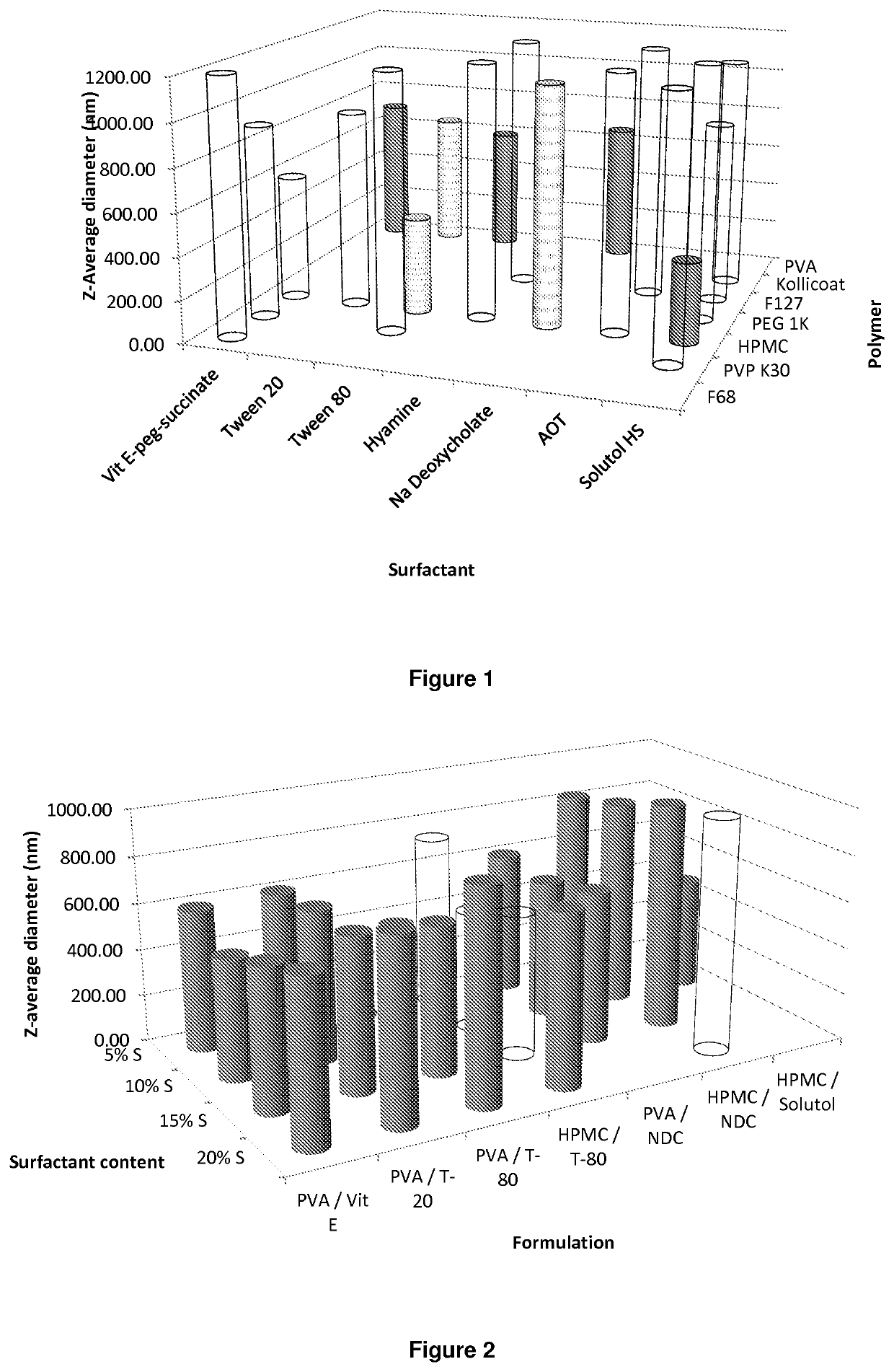
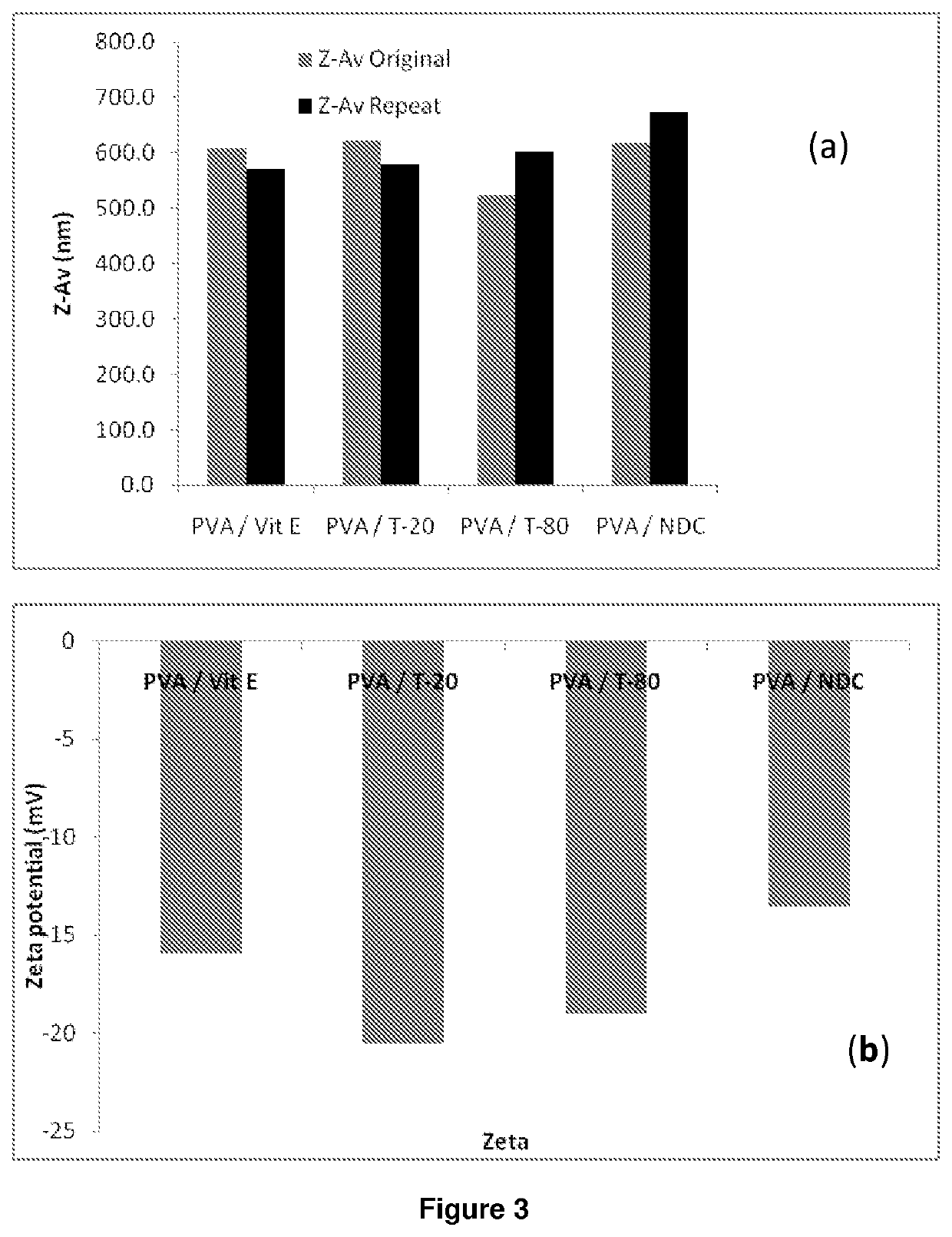
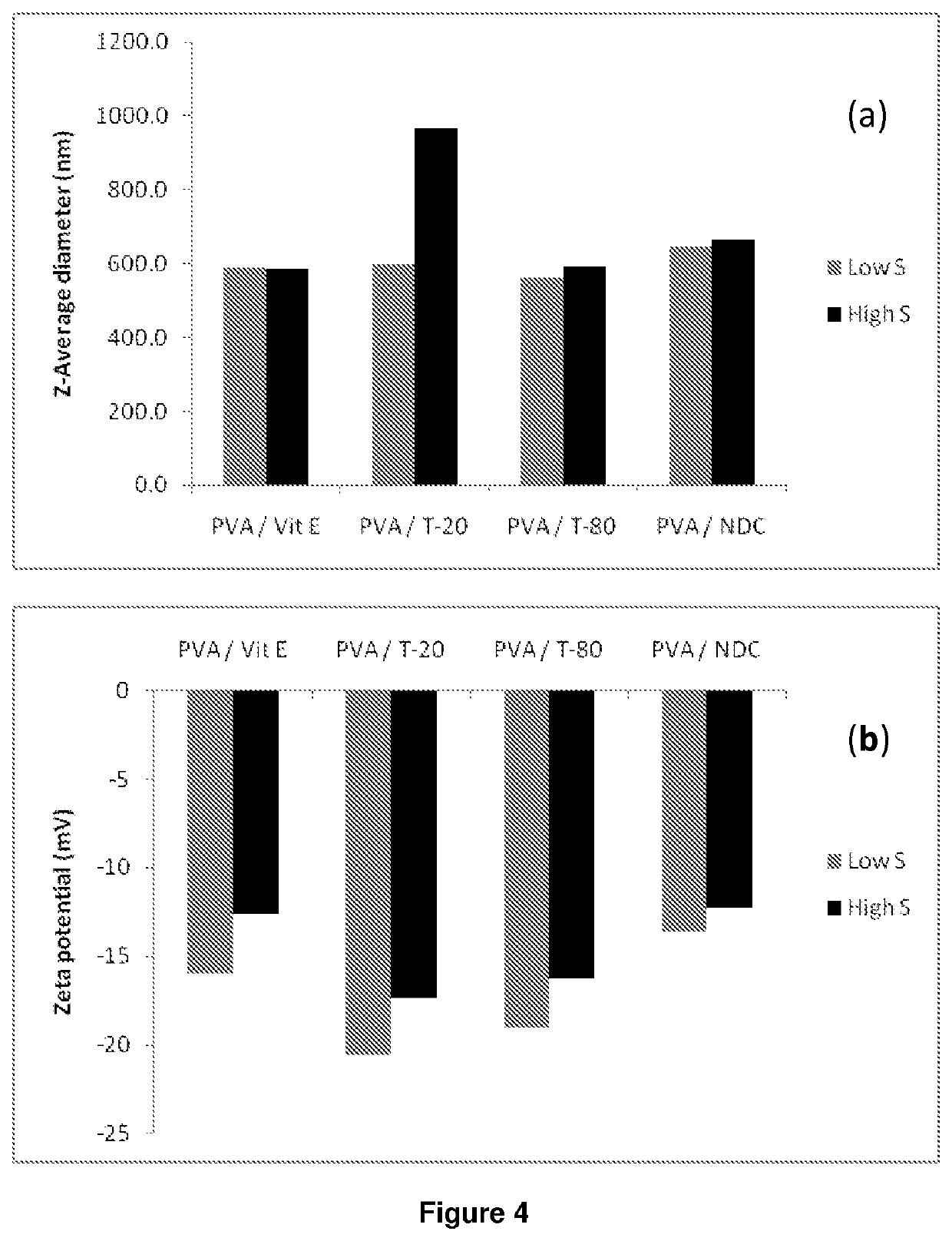
ActiveUS20140212501A1High drug loadingAdvanced dosage formPowder deliveryOrganic active ingredientsHydrophilic polymersRetroviral infection
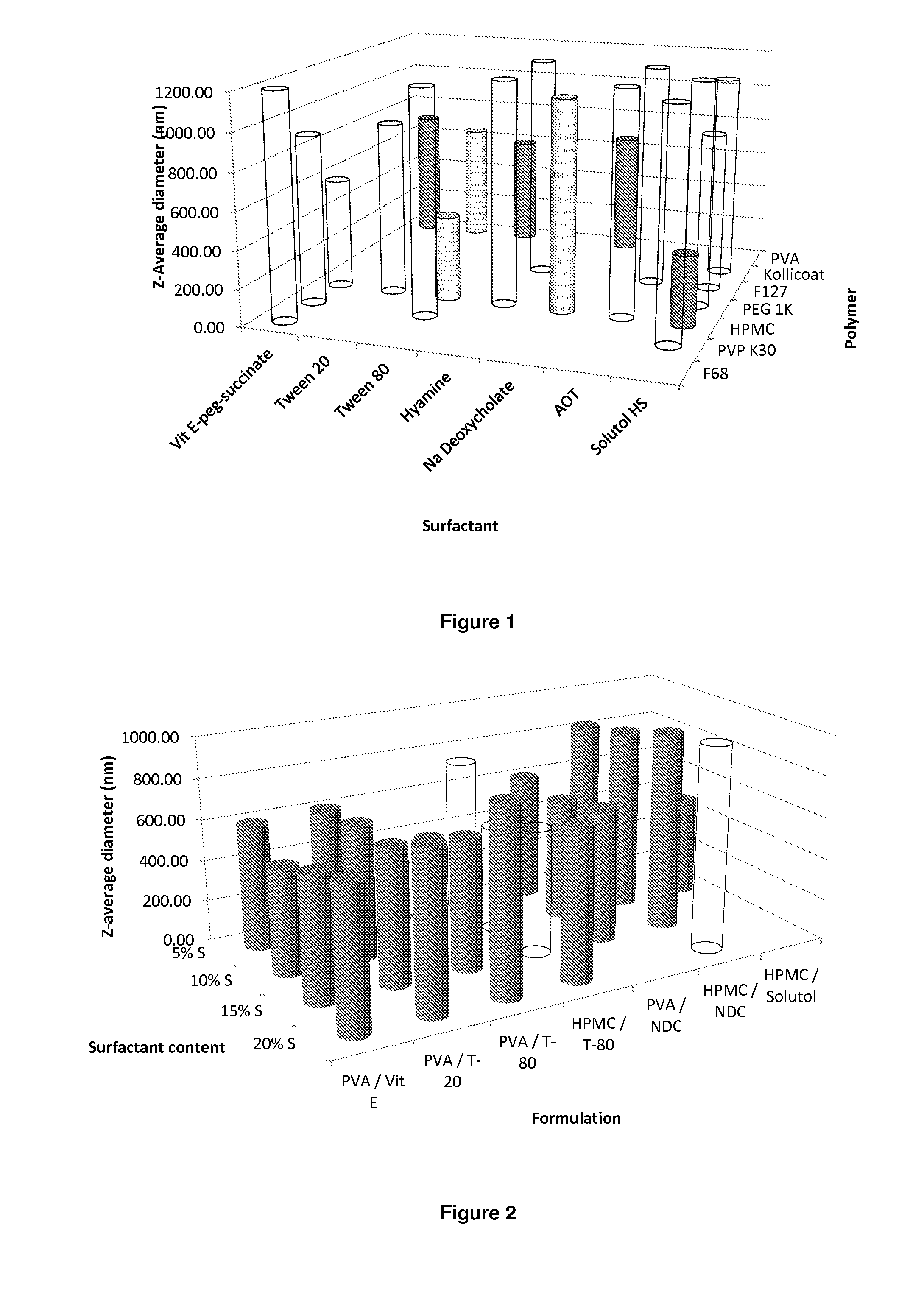
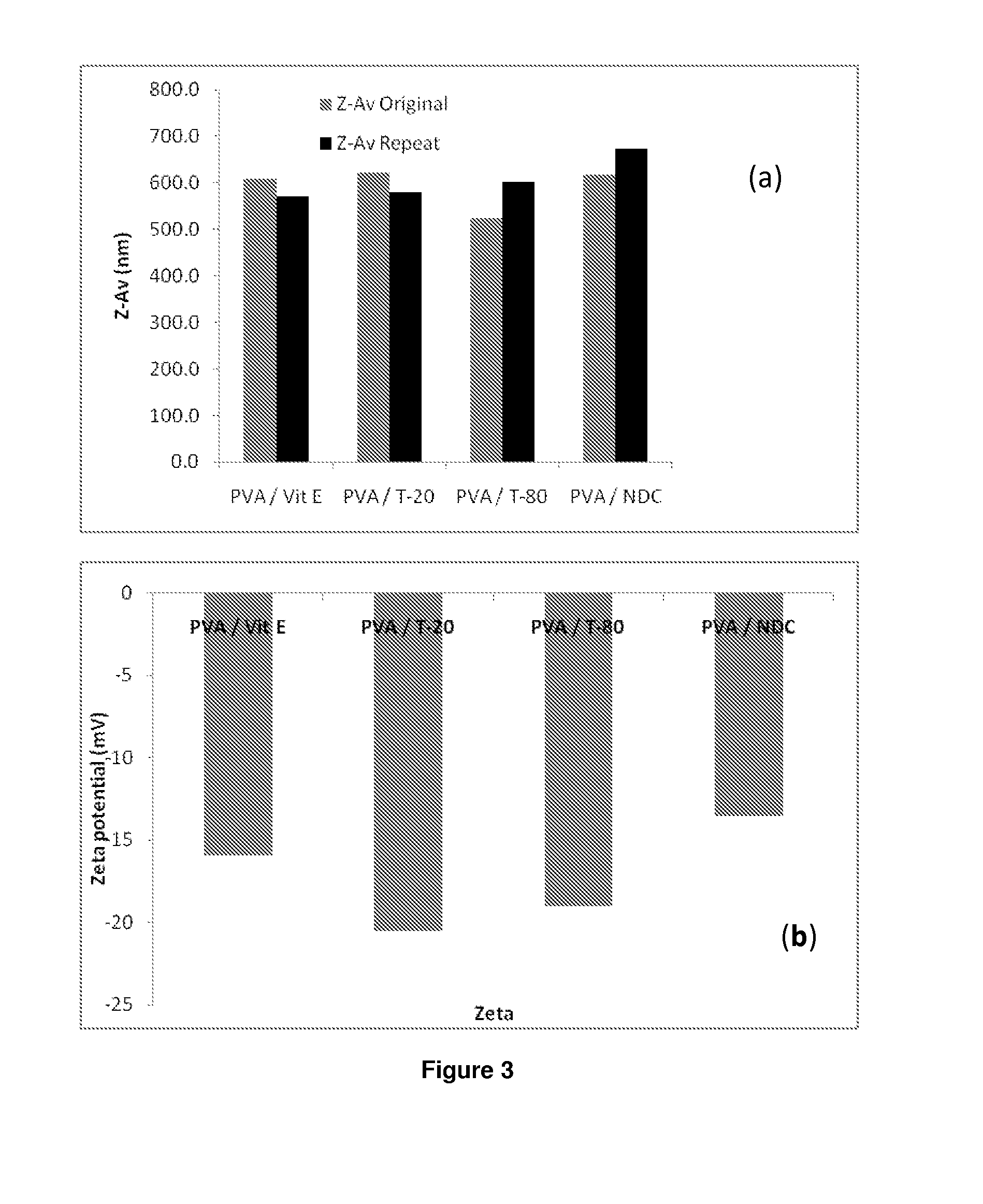
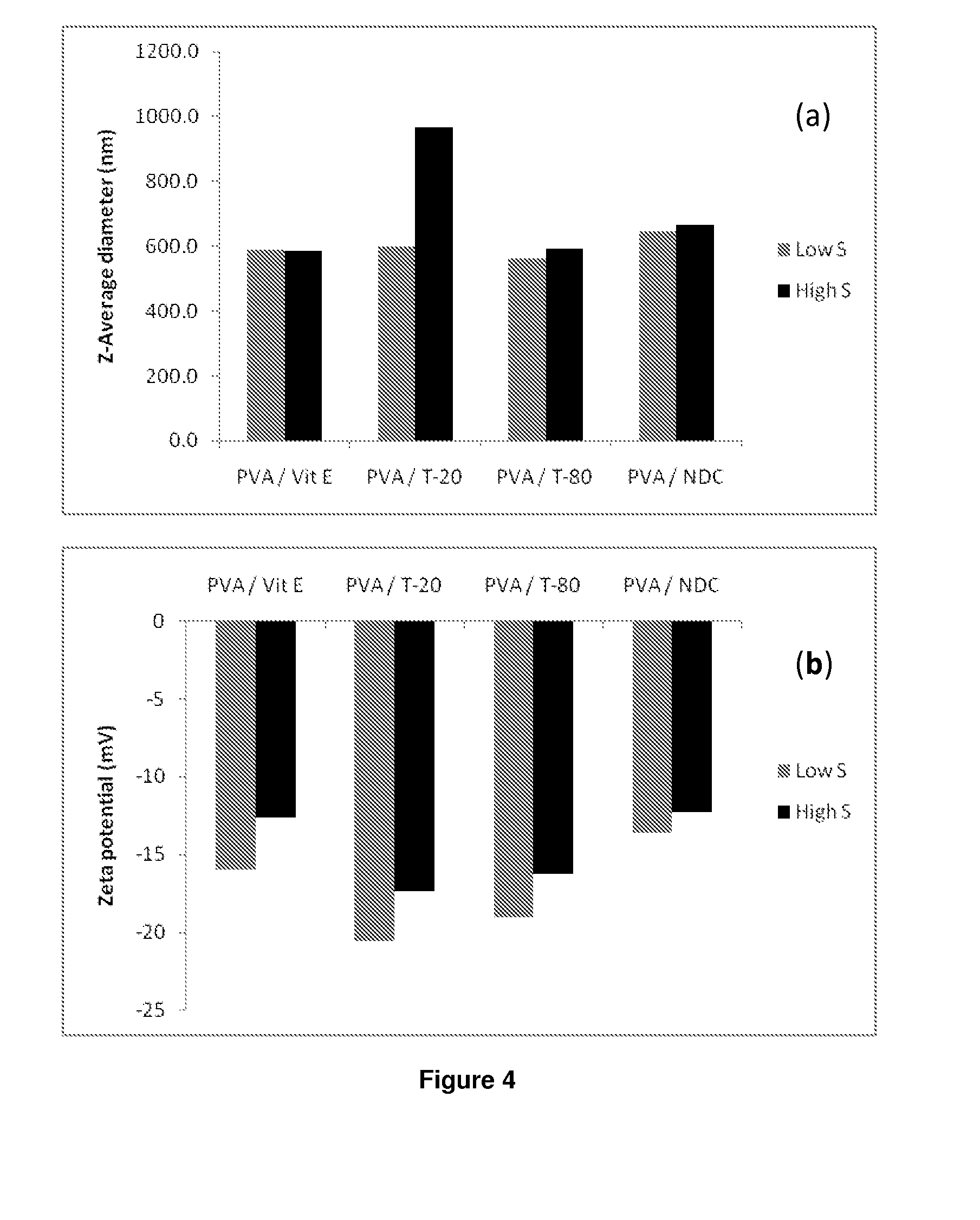
The present invention relates to a solid composition and an aqueous dispersion comprising nanoparticles of the anti-retroviral drug lopinavir. The solid composition and aqueous dispersion additionally comprise a mixture of a hydrophilic polymer and a surfactant. The surfactant is selected from vitamin-E-polyethylene glycol-succinate (Vit-E-PEG-succinate), a polyoxyethylene sorbitan fatty acid ester, N-alkyldimethylbenzylammonium chloride, sodium deoxycholate, dioctyl sodium sulfosuccinate, polyethyleneglycol-12-hydroxystearate, polyvinyl alcohol (PVA), and a block copolymer of polyoxyethylene and polyoxypropylene, or a combination thereof. The hydrophilic polymer is suitably selected from polyvinyl alcohol (PVA), a polyvinyl alcohol-polyethylene glycol graft copolymer, a block copolymer of polyoxyethylene and polyoxypropylene, polyethylene glycol, hydroxypropyl methyl cellulose (HPMC), and polyvinylpyrrolidone, or a combination thereof. The present invention also relates to processes for preparing both the solid composition and the aqueous dispersion, as well as to their use in therapy for the treatment and / or prevention of retroviral infections such as human immunodeficiency virus (HIV).
Owner:UNIV OF LIVERPOOL
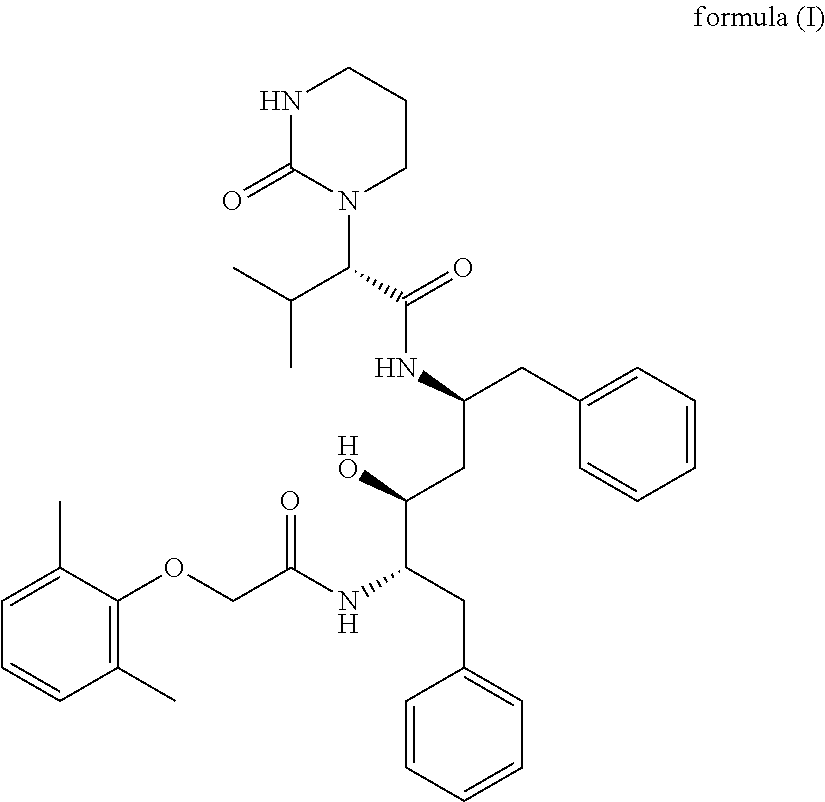
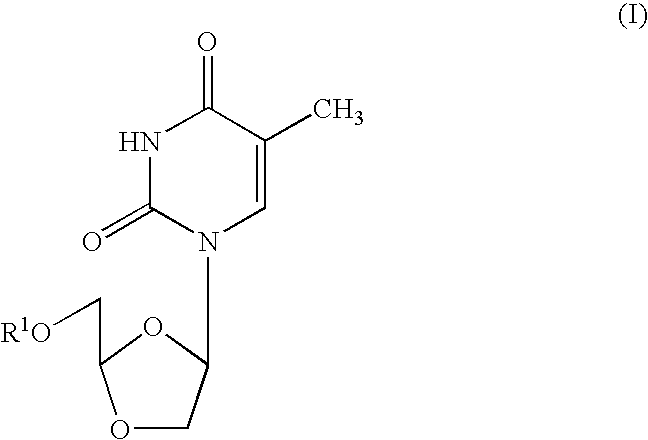
Novel method for preparing lopinavir
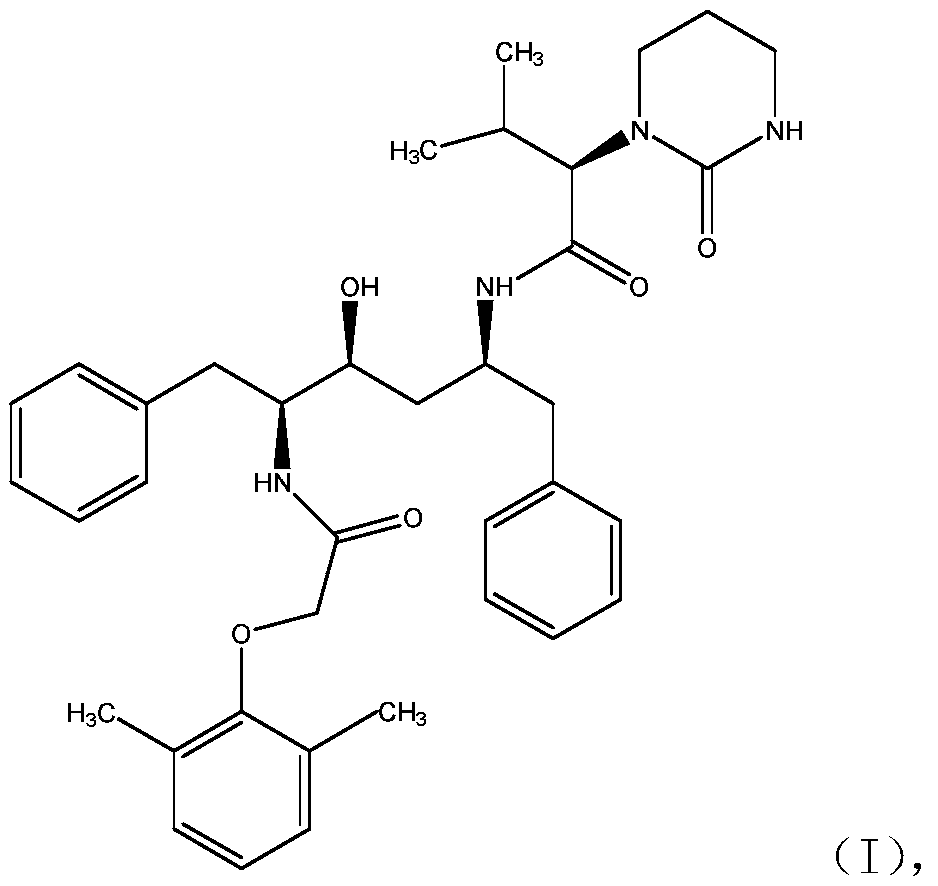
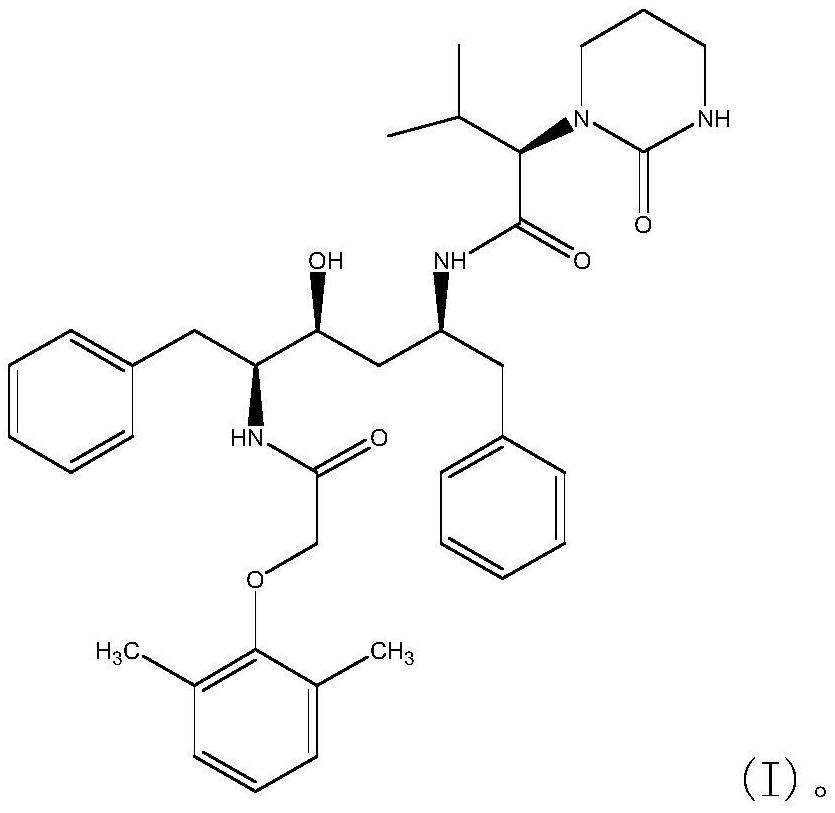
ActiveCN111018791AFew synthetic stepsCondensation reaction conditions are mildOrganic chemistryChemical compoundCombinatorial chemistry
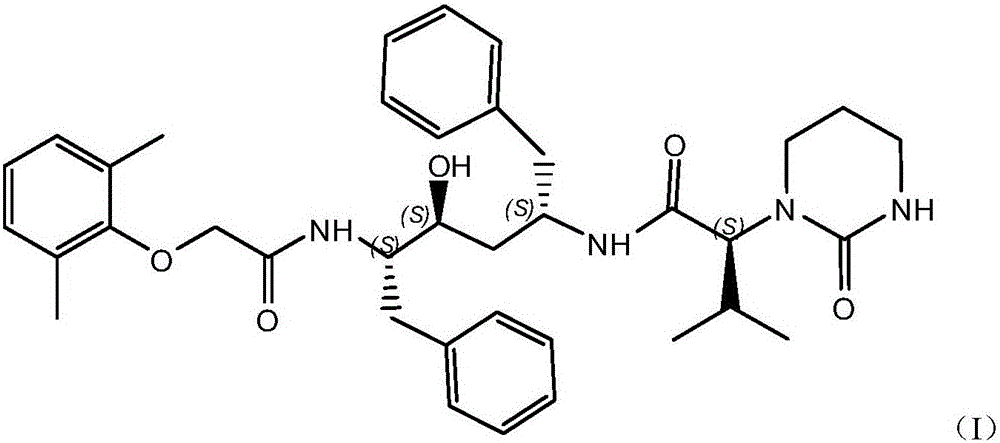
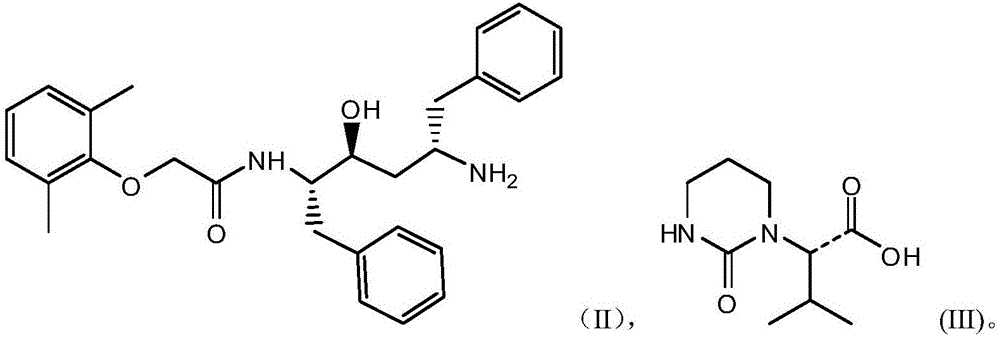
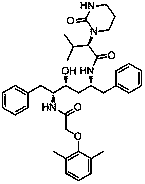
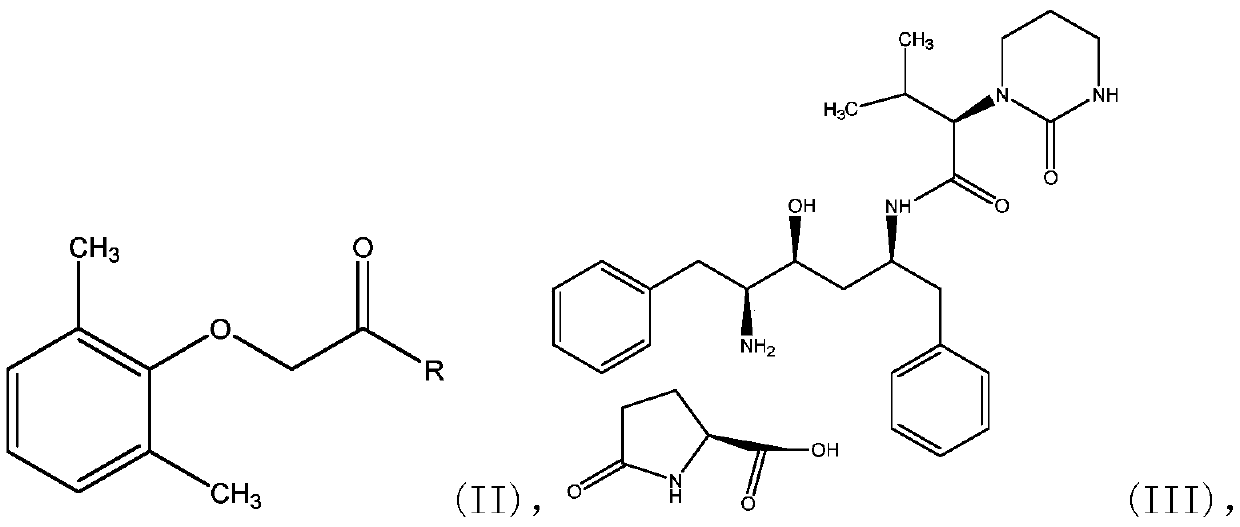
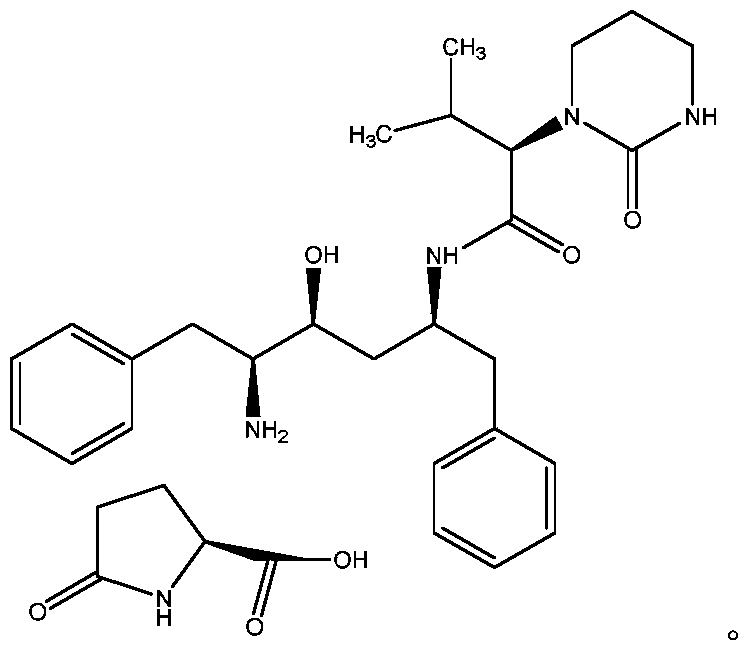
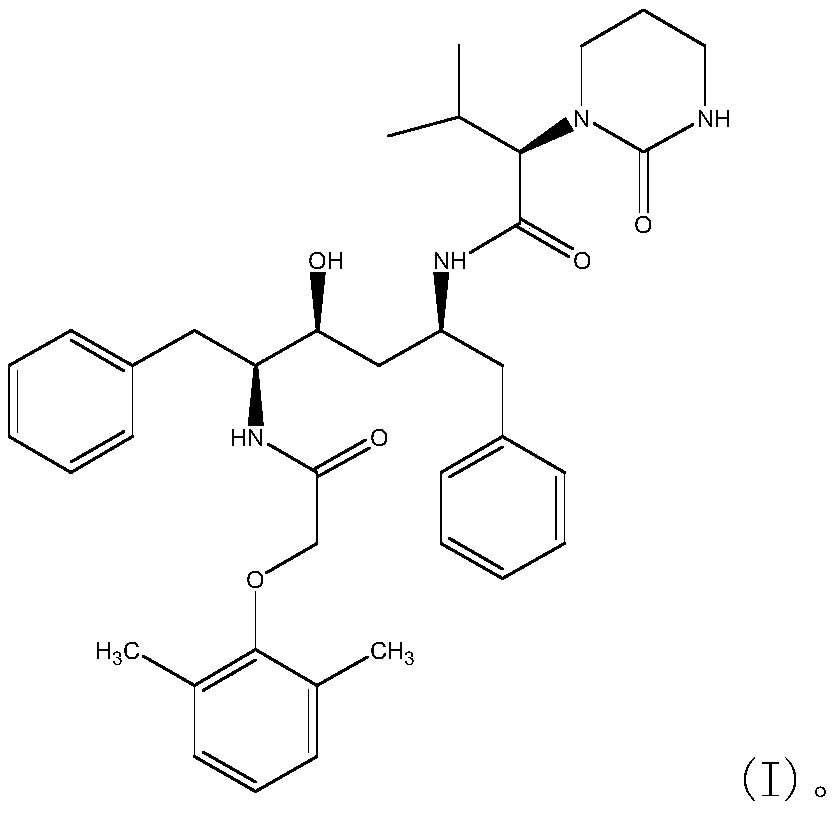
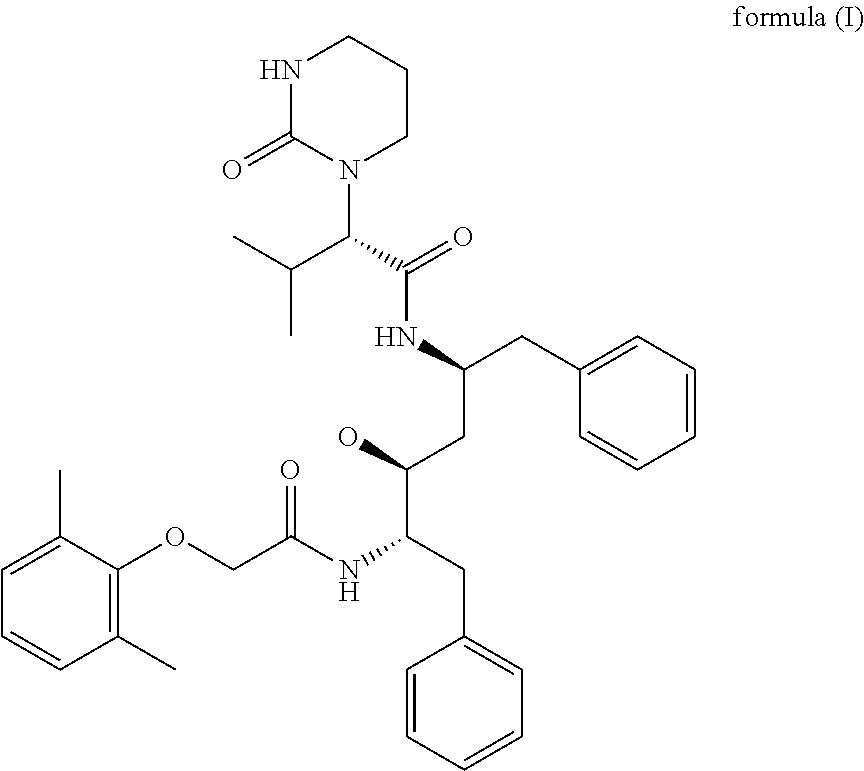
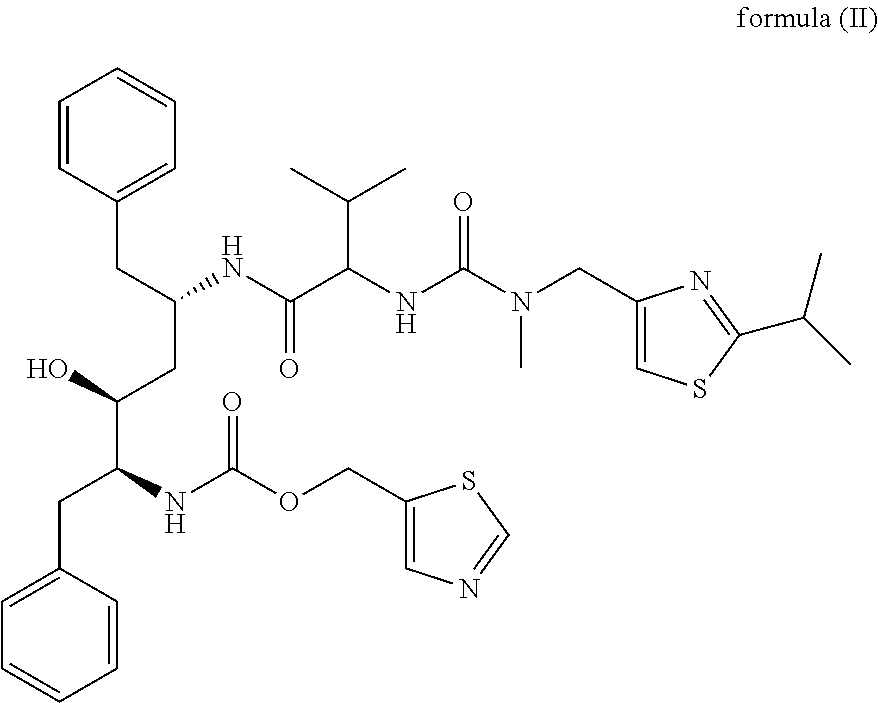
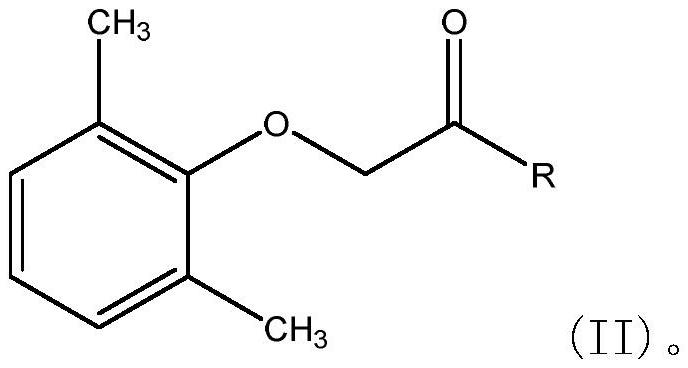
The invention relates to a novel method for preparing lopinavir (I), a condensation reaction of a compound shown in a formula (II) and a compound shown in a formula (III) is carried out at room temperature under mild condensation reaction conditions, and heating or cooling is not needed; the preparation method does not need special chemical reagents, the special chemical reagents are solvents andreagents commonly used in laboratories, the total yield is higher than 85%, preferably higher than 90%, in some embodiments, the yield reaches 96%, and the preparation method is especially suitable for industrial production.
Owner:厦门蔚嘉制药有限公司
Preparation of lopinavir by one-pot method
ActiveCN110903249AFew synthetic stepsCondensation reaction conditions are mildOrganic chemistry methodsCarboxyl radicalOrganic solvent
The invention relates to preparation of lopinavir as shown in formula (I) by a one-pot method. 2, 6-dimethylphenoxyacetic acid, a carboxyl activator and a compound as shown in a formula (III) are subjected to a condensation reaction in an organic solvent under a mild condensation reaction condition, the reaction can be carried out at room temperature without heating or cooling. The preparation method does not need special chemical reagents, and the solvent and the reagents used in the method are commonly used in laboratories. The total yield is higher than 85%, preferably higher than 90%, andthe yield reaches 96% in some embodiments. The preparation method is especially suitable for industrial production.
Owner:厦门蔚嘉制药有限公司
Compositions of lopinavir and ritonavir
ActiveUS9532979B2Optimize allocationPromote accumulationPowder deliveryOrganic active ingredientsRetroviral infectionPolyethylene glycol
The present inventions relates to a solid composition and an aqueous dispersion comprising nanoparticles of the anti-retroviral drugs lopinavir and ritonavir. The solid composition and aqueous dispersion additionally comprise a mixture of a hydrophilic polymer and a surfactant. The surfactant is selected from vitamin-E-polyethylene glycol-succinate (Vit-E-PEG-succinate), a polyoxyethylene sorbitan fatty acid ester, N-alkyldimethylbenzylammonium chloride, sodium deoxycholate, dioctyl sodium sulfosuccinate, polyethyleneglycol-12-hydroxystearate, polyvinyl alcohol (PVA), and a block copolymer of polyoxyethylene and polyoxypropylene, or a combination thereof. The hydrophilic polymer is suitably selected from polyvinyl alcohol (PVA), a polyvinyl alcohol-polyethylene glycol graft copolymer, a block copolymer of polyoxyethylene and polyoxypropylene, polyethylene glycol, hydroxypropyl methyl cellulose (HPMC), and polyvinylpyrrolidone, or a combination thereof. The present invention also relates to processes for preparing both the solid composition and the aqueous dispersion, as well as to their use in therapy for the treatment and / or prevention of retroviral infections such as human immunodeficiency virus (HIV).
Owner:UNIV OF LIVERPOOL
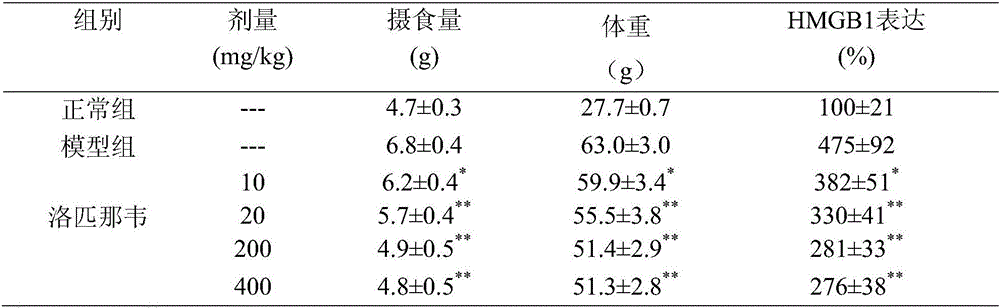
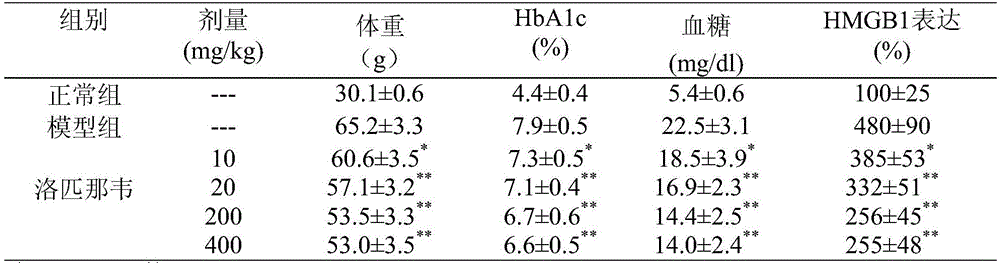
Lopinavir used for preparing medicine for guarding against or treating ischemic cardiovascular and cerebrovascular disease
The invention relates to the new application of medicine and particularly relates to lopinavir which can be used for guarding against or treating ischemic cardiovascular and cerebrovascular diseases through restraining the expression of HMG (High Mobility Group Protein) B1. According to the invention, the dosage range is 120 mg-6000 mg and 120-3000 mg of lopinavir is particularly preferred.
Owner:BINZHOU MEDICAL COLLEGE
Micro-particulated nanocapsules containing lopinavir with enhanced oral bioavailability and efficacy
InactiveUS20160235749A1Improve absorption and controlled-releaseControl releaseOrganic active ingredientsGranular deliveryControl releaseActive agent
The present disclosure provides controlled-release delivery systems for oral delivery of active agents, e.g. lopinavir, comprising micro-particulated with enhanced oral bioavailability and efficacy, which may be used for treating HIV.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Lopinavir and ritonavir compound tablet and preparation method thereof
ActiveCN112263554ASimple prescriptionAvoid introducingOrganic active ingredientsAntiviralsActive agentHot melt
The invention relates to a lopinavir and ritonavir compound tablet. The tablet comprises lopinavir, ritonavir, a dispersion carrier material, a flow aid, a surfactant and other additional auxiliary materials. A hot-melt extrusion mode is adopted for preparation. A preparation method comprises the following steps of uniformly mixing the bulk drugs lopinavir and ritonavir, the dispersion carrier material and the flow aid according to the prescription dosage to prepare a mixed material, crushing or grinding the mixed material until D90 is 100-150 [mu]m, performing extrusion through a hot melt extruder, and adding the auxiliary materials to prepare the tablet. The hot melt extrusion temperature is 90-110 DEG C. The prepared compound tablet is good in dissolution rate and uniform in content, and the components of the two effective medicines can be synchronously released.
Owner:ANHUI BIOCHEM BIO PHARMA
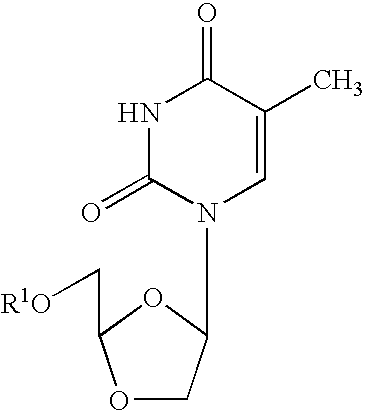
Dioxolane thymine and combinations for use against 3tc/azt resistant strains of hiv
InactiveUS20050209196A1Inhibits growth and replication and elaborationBiocideSugar derivativesDelavirdinePhosphate
The present invention relates to the use of a dioxolane thymine compound according to the chemical structure of Formula (I): where R1 is H, an acyl group, a C1-C20 alkyl or ether group, a phosphate, diphosphate, triphosphate or phosphodiester group, for use in the treatment of HIV infections which exhibit resistance to 3TC and / or AZT. Preferably, compounds according to the present invention are combined with at least one anti-HIV agent which inhibits HIV by a mechanism other than through the inhibition of thymidine kinase (TK). These agents include those selected from among nucleoside reverse transcriptase inhibitors (NRTI), non-nucloeoside reverse transcriptase inhibitors, protease inhibitors, fusion inhibitors, among others. These agents are generally selected from the group consisting of 3TC (Lamivudine), AZT (Zidovudine), (−)-FTC, ddI (Didanosine), ddC (zalcitabine), abacavir (ABC), tenofovir (PMPA), D-D4FC (Reverset), D4T (Stavudine), Racivir, L-D4FC, NVP (Nevirapine), DLV (Delavirdine), EFV (Efavirenz), SQVM (Saquinavir mesylate), RTV (Ritonavir), IDV (Indinavir), SQV (Saquinavir), NFV (Nelfinavir), APV (Amprenavir), LPV (Lopinavir), fuseon and mixtures thereof. The TK dependent agents, such as AZT and D4T, may be used in combination with one of the dioloxane thymine compounds according to the present invention, but the use of such agents may be less preferred. In preferred compositions according to the present invention, R1 is preferably H or a C2-C18 acyl group or a monophosphate group. Pharmaceutical compositions and methods of reducing the likelihood that a patient at risk for contract an HIV infection will contract the infection are other aspects of the present invention.
Owner:UNIV OF GEORGIA RES FOUND INC +1
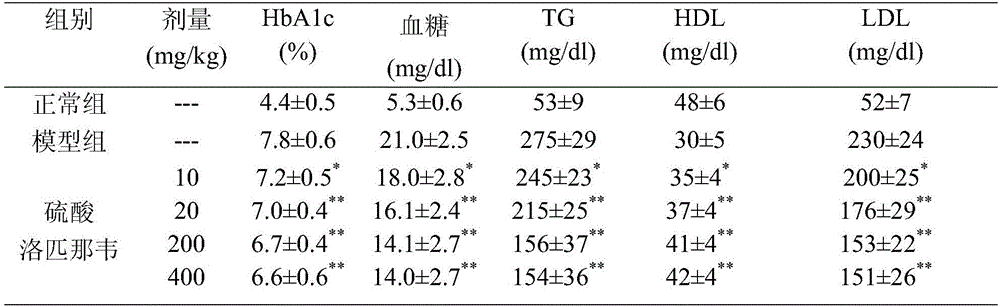
New application of lopinavir to medicines
The invention provides a new application of lopinavir to medicines and in particular relates to an application of lopinavir to medicines for preventing or treating obesity, type II diabetes, diabetic nephropathy and non-alcoholic fatty liver diseases. The oral dosage of lopinavir ranges between 50mg and 2000mg in application, preferably 50-1000mg.
Owner:BINZHOU MEDICAL COLLEGE
Oral suspension preparation containing lopinavir and ritonavir
InactiveCN108066344AGuaranteed decentralizationEasy to useOrganic active ingredientsAntiviralsOral suspensionsPatient compliance
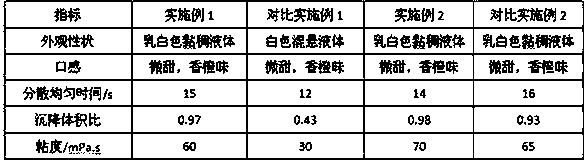
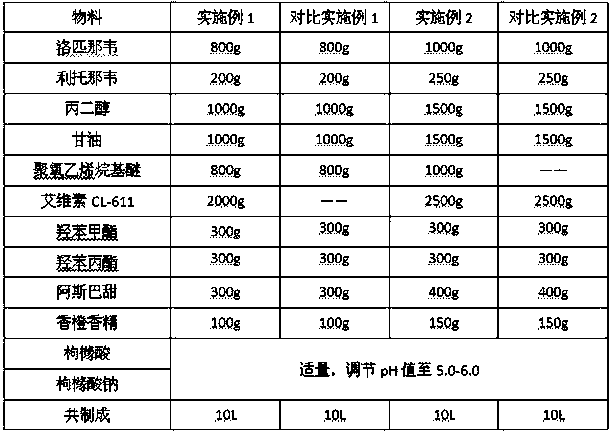
The invention provides a compound oral suspension containing lopinavir and ritonavir and a preparation method thereof. The oral suspension comprises lopinavir and ritonavir active ingredients and pharmaceutically acceptable auxiliary materials. The lopinavir and ritonavir compound oral suspension provided by the invention is stable in quality, cannot be settled and caked after storing for a long period of time, can be quickly and evenly dispersed after shaking, and has good taste, and good patient compliance. The lopinavir and ritonavir compound oral suspension provided by the invention has high dissolution rate and improvement on bioavailability. The preparation provided by the invention is simple in preparation method and is suitable for industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Novel medicine use of lopinavir in alleviating toxicity of irinotecan
The present invention provides anti-HIV protease inhibitor lopinavir alone or the combination of lopinavir and ritonavir in the prevention or / and treatment of delayed diarrhea caused by an antineoplastic drug, diarrhea caused intestinal Use in drugs for dysfunction, wherein the antineoplastic drug refers to irinotecan or its active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). In the present invention, lopinavir has clear pharmacological effects, can effectively alleviate delayed diarrhea, and protect intestinal function.
Owner:BINZHOU MEDICAL COLLEGE
Compositions comprising lopinavir and treatment of conditions
PendingCN113924097AInhibit progressOrganic active ingredientsAerosol deliveryPharmaceutical drugHerpes simplex virus infection
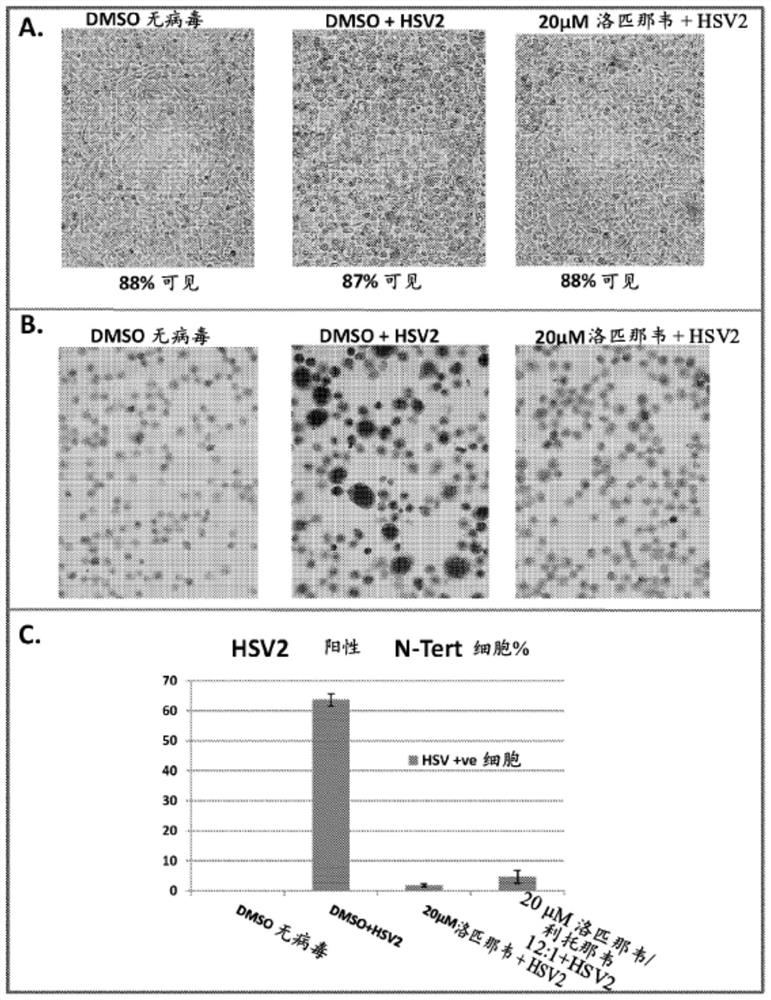
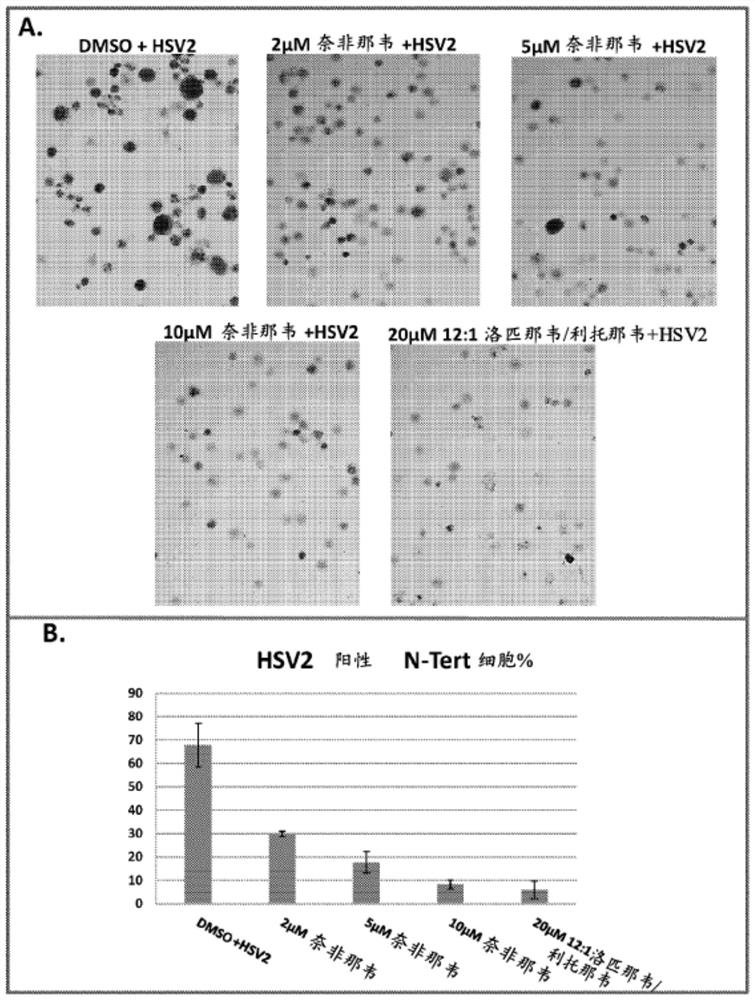
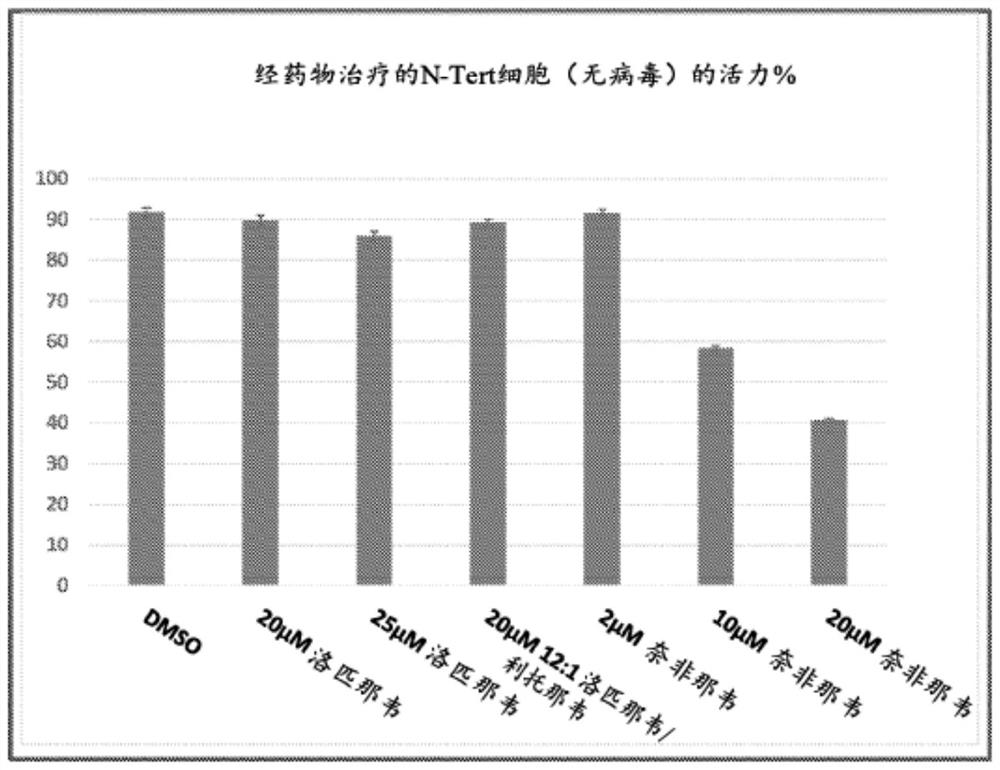
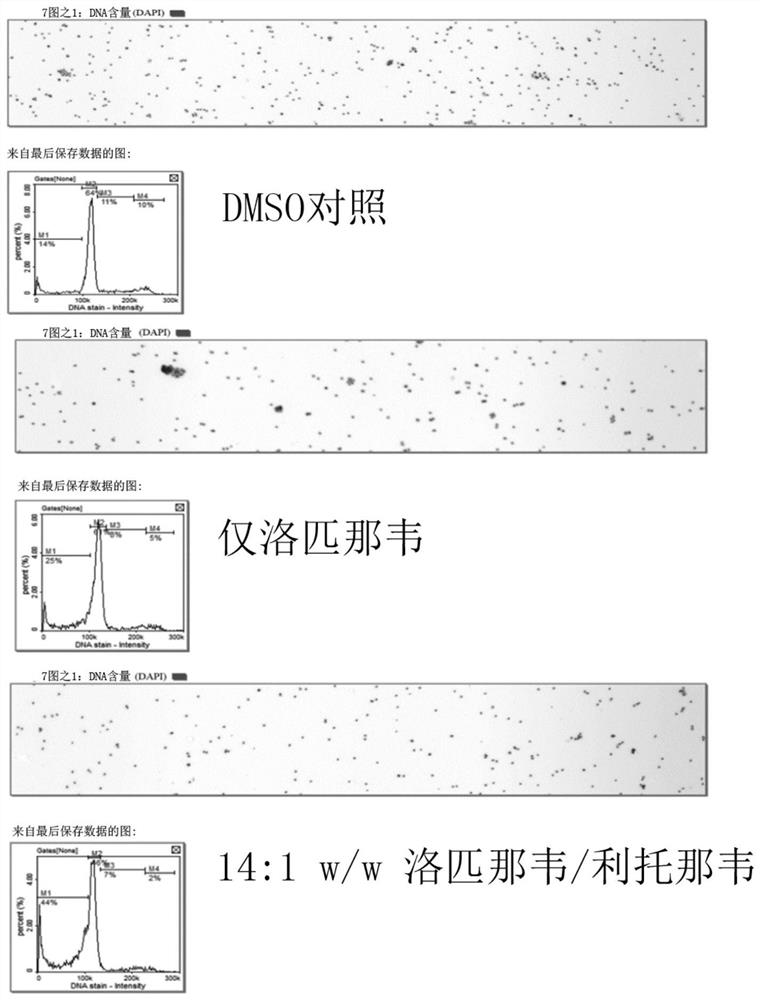
Provided herein are methods and compositions for treating and / or inhibiting the development or progression of conditions caused by, or associated with, Herpes Simplex Virus (HSV) infection. In particular, provided are compositions comprising lopinavir alone, or lopinavir and ritonavir, methods for their manufacture; and the use of said pharmaceutical compositions as a medicament. In particular, pharmaceutical compositions are provided for use in treating and / or inhibiting the progression of HSV related conditions.
Owner:DOUGLAS PHARMA
Treatments
PendingCN112165941AOrganic active ingredientsOintment deliveryPharmaceutical drugPharmaceutical medicine
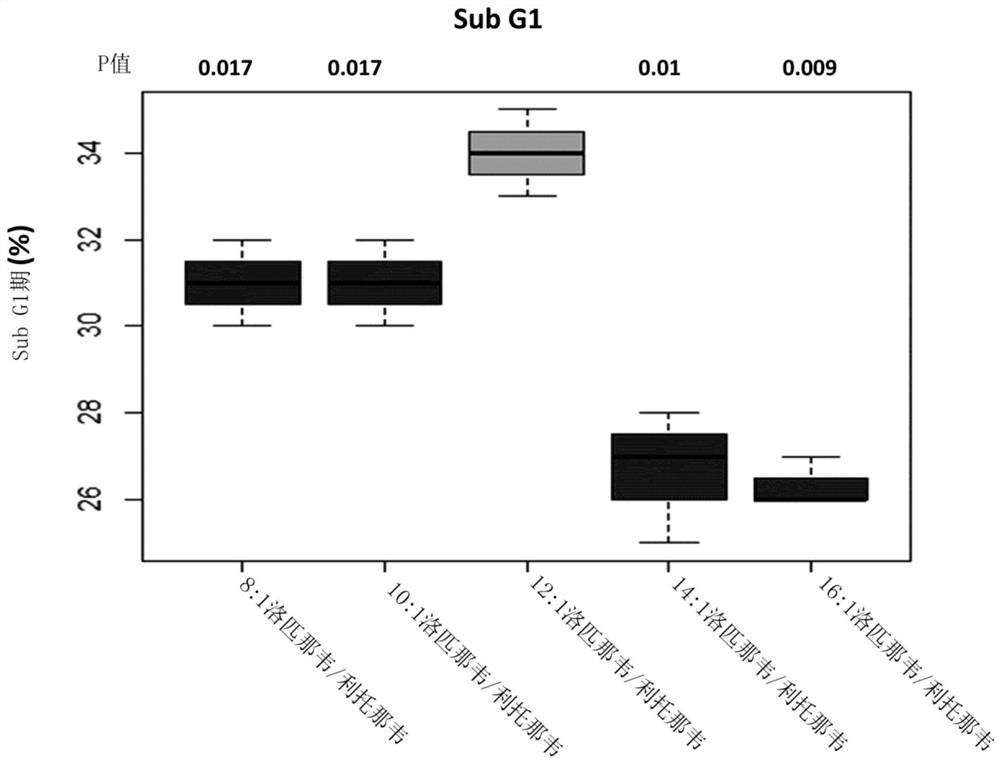
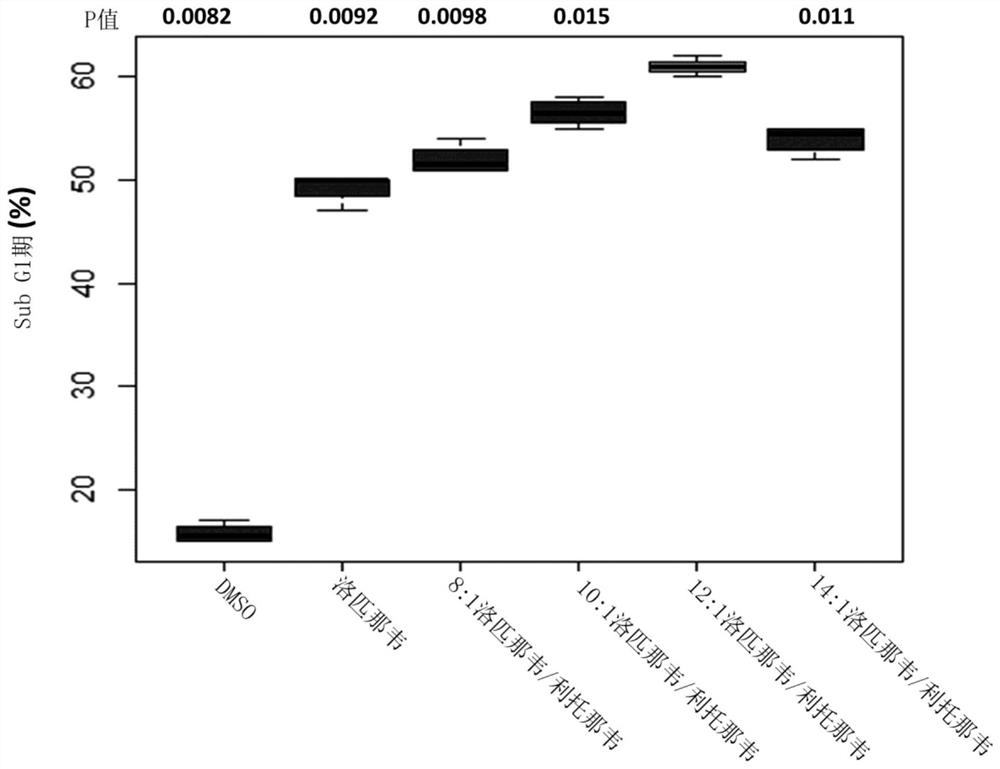
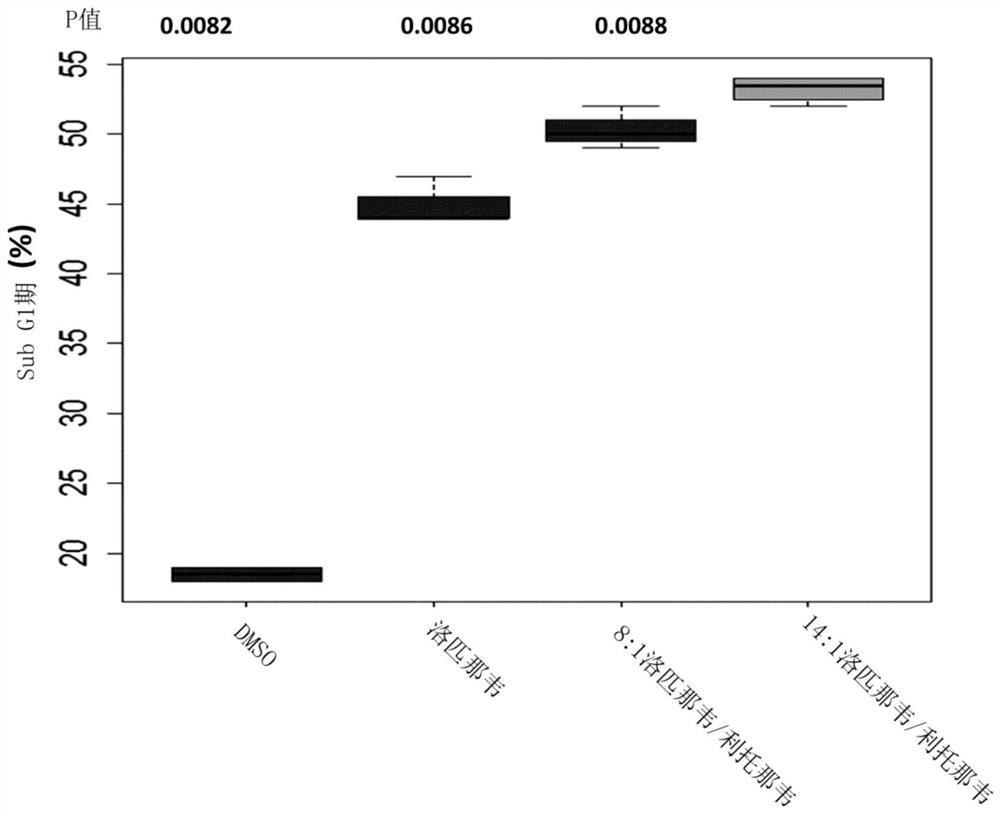
The present invention concerns pharmaceutical compositions formulated for dermal application comprising a therapeutically effective amount of lopinavir and ritonavir in a pharmaceutically acceptable vehicle and wherein the weight ratio (w / w) of lopinavir: ritonavir is between 9:1 and 18:1. Such compositions are useful for treating, or preventing, skin cancers and premalignant dermal conditions.
Owner:DOUGLAS PHARMA
Dosage form comprising lopinavir and ritonavir
InactiveUS9370578B2Good content uniformityMaintain good propertiesOrganic active ingredientsPeptide/protein ingredientsActive agentDosage form
The present invention relates to an oral dosage form comprising crystalline lopinavir and crystalline ritonavir. The invention further relates to methods of preparing said oral dosage forms containing the above pharmaceutical active agents.
Owner:RATIOPHARM GMBH
Lopinavir and ritonavir for the treatment of cervix disorders
The present invention concerns pharmaceutical compositions formulated for topical application that are useful in the treatment of HPV-related pathologies and in particular the treatment of premalignant and malignant conditions of the cervix. The compositions comprise a therapeutically effective amount of lopinavir and ritonavir in a pharmaceutically acceptable vehicle and wherein the weight ratio(w / w) of lopinavir: ritonavir is between 9:1 and 18:1.
Owner:DOUGLAS PHARMA
Compositions of lopinavir
ActiveUS10603279B2Advanced dosage formImprove distributionPowder deliveryOrganic active ingredientsHydrophilic polymersPolyvinyl alcohol
Owner:UNIV OF LIVERPOOL
One-pot preparation of lopinavir
ActiveCN110903249BFew synthetic stepsCondensation reaction conditions are mildOrganic chemistry methodsPolymer scienceCarboxyl radical
One-pot preparation of lopinavir (I), which is performed by 2,6-dimethylphenoxyacetic acid, a carboxyl activator, and a compound represented by formula (III) in an organic solvent to carry out a condensation reaction. Condensation reaction conditions are mild, in It can be carried out at room temperature without heating or cooling; the preparation method does not require special chemical reagents, and all are solvents and reagents commonly used in laboratories, and the total yield is higher than 85%, preferably higher than 90%, and in some embodiments, the yield Up to 96%, especially suitable for industrial production.
Owner:厦门蔚嘉制药有限公司
Novel tenofovir disoproxil salt and the preparation method thereof
InactiveCN105452249AImprove stabilityGood light stabilityOrganic chemistry methodsAntiviralsEmtricitabineBULK ACTIVE INGREDIENT
The present invention relates to a novel tenofovir disoproxil salt and the preparation method thereof. The crystalline form of tenofovir disoproxil orotate according to the present invention is an anhydride and it has superior physicochemical properties that are useful in pharmaceutical preparation as it resolves problems caused by physicochemical properties of tenofovir disoproxil fumarate. Namely, the crystalline form of tenofovir disoproxil orotate according to the present invention has a better than equal light stability compared to tenofovir disoproxil fumarate and has a superior stability especially under extreme stress conditions. Additionally, it is neither corrosive nor harmful to human body, thus making it safe for preparation and easy to manage. All of these characteristics make this invention useful for mass production. The present invention also provides a preparation method with and without using organic solvent, thus providing an industrially applicable and environment-friendly preparation method. Furthermore, tenofovir disoproxil orotate prepared according to the present invention can be used as an antiviral treating agent and can also be made into an anti-HIV treating composition if mixed with other drugs that have anti-HIV activity. The composition can be made into mixtures with one to six of the following other anti-HIV active ingredient medications that include Emtricitabine, Efavirenz, Rilpivirenz, Cobicistat, Elvitegravir, Raltegravir, Ritonavir, Lopinavir, Darunavir, Atazanavir and salts thereof for the treatment of HIV infection.
Owner:DONG A ST CO LTD
A kind of lopinavir and ritonavir compound high homogeneity nano co-dispersion and preparation method thereof
ActiveCN103655571BSimple manufacturing methodSuitable for industrial productionOrganic active ingredientsAntiviralsPharmaceutical industryDissolution
The invention provides a lopinavir and ritonavir compound high-uniformity nano co-dispersion body. The lopinavir and ritonavir compound high-uniformity nano co-dispersion body consists of lopinavir and ritonavir which are taken as active ingredients, a dispersing aid, an anti-agglormeration material, a dispersing stabilizer, a dispersion supporter and a lubricant. The lopinavir and ritonavir which are taken as active ingredients account for 25%-60%. The lopinavir and ritonavir compound high-uniformity nano co-dispersion body prepared by the preparation method disclosed by the invention can be prepared into a single-dose preparation according to a normal method. According to a dissolution rate experiment, the preparation disclosed by the invention has release amount of 40%-80% within 30 minutes. The preparation prepared by the dispersion body disclosed by the invention is high in dissolution rate, and can be used for improving the bioavailability. The lopinavir and ritonavir compound high-uniformity nano co-dispersion body prepared by the preparation method disclosed by the invention satisfies and is superior to mixing uniformity standards. The preparation method disclosed by the invention is simple and easy to implement, can be used for popularizing application of a nano mechanical grinding technology on the pharmaceutical industry and promoting the development of the technology, and is suitable for industrial production and greater in application value.
Owner:SHANGHAI SUNTECH PHARMA +1
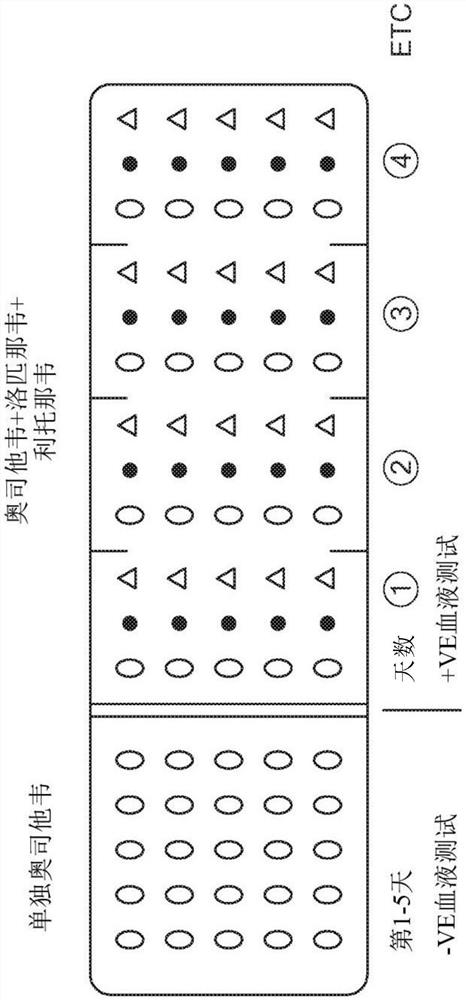
Product of manufacture and method for treating, ameliorating or preventing coronavirus infection
Provided are therapeutic agents, combination therapies, and methods for the treatment of coronavirus infections and related diseases, particularly COVID-19. The therapeutic agents provided include oseltamivir, lopinavir, ritonavir, chloroquine or hydroxychloroquine, azithromycin, clarithromycin, doxycycline, ivermectin, and combinations thereof.
Owner:TOPELIA AUSTRALIA PTY LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com