Treatments
A composition and drug technology, applied in the field of treatment, can solve problems such as skin conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
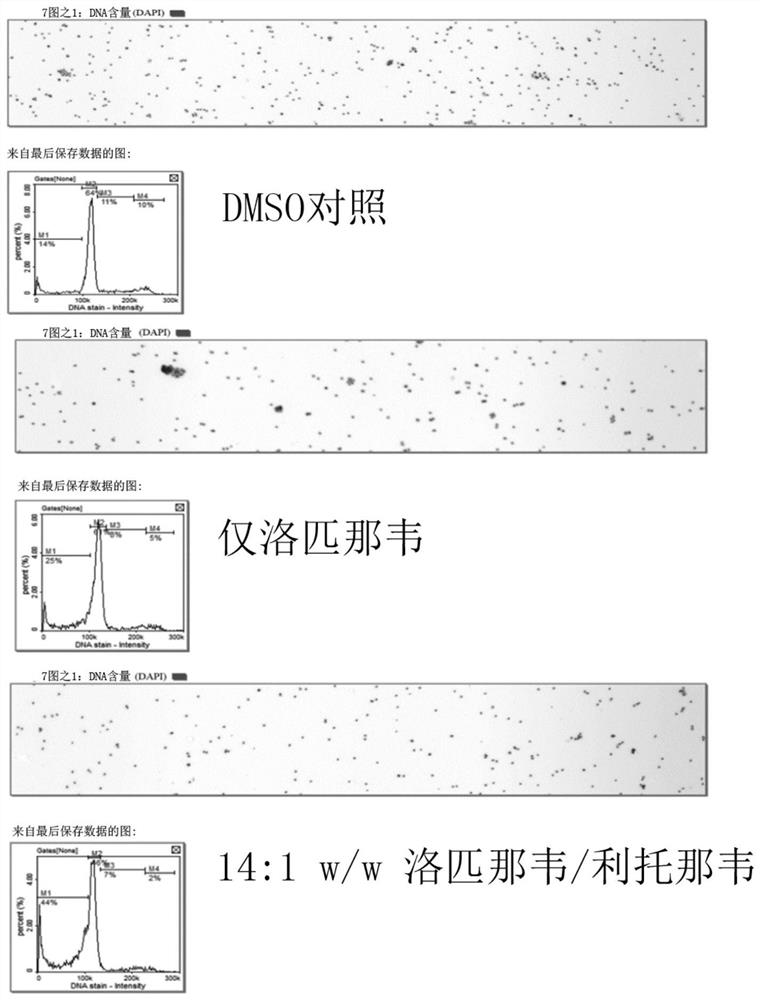
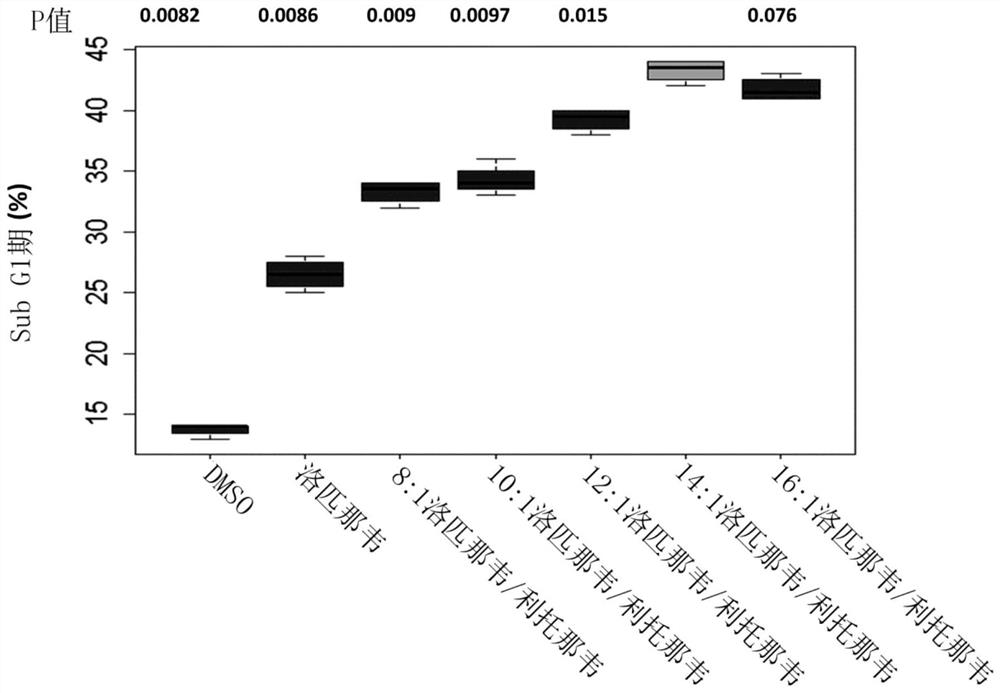
[0210] Example 1: Evaluation of the effect of lopinavir:ritonavir at a total API concentration of 20 μM in a ratio of 8:1-16:1 w / w on hTERT cells
[0211] N-TERT cells are an immortal, untransformed cell line derived from differentiated cells with wild-type p53 and normal karyotype. They proliferate indefinitely and can be transformed by a variety of treatments. Therefore, these cells represent a good model to evaluate the effect of lopinavir and ritonavir on AK, an untransformed and dysplastic precancerous lesion. N-TERT cells derived from neonatal foreskin keratinocytes were used in these experiments as described by Dickson et al. (2000) (Mol Cell Biol Feb 2000 p1436-1447).
[0212] 1.1 Method
[0213] 1.1.1 Cell culture
[0214] at 5% CO 2 N-TERT cells were cultured by standard methods in keratinocyte serum-free medium (KSFM) supplemented with bovine pituitary extract (BPE) and epidermal growth factor (EGF) at and 37°C.
[0215] Experiments were performed on cells inoc...
Embodiment 2
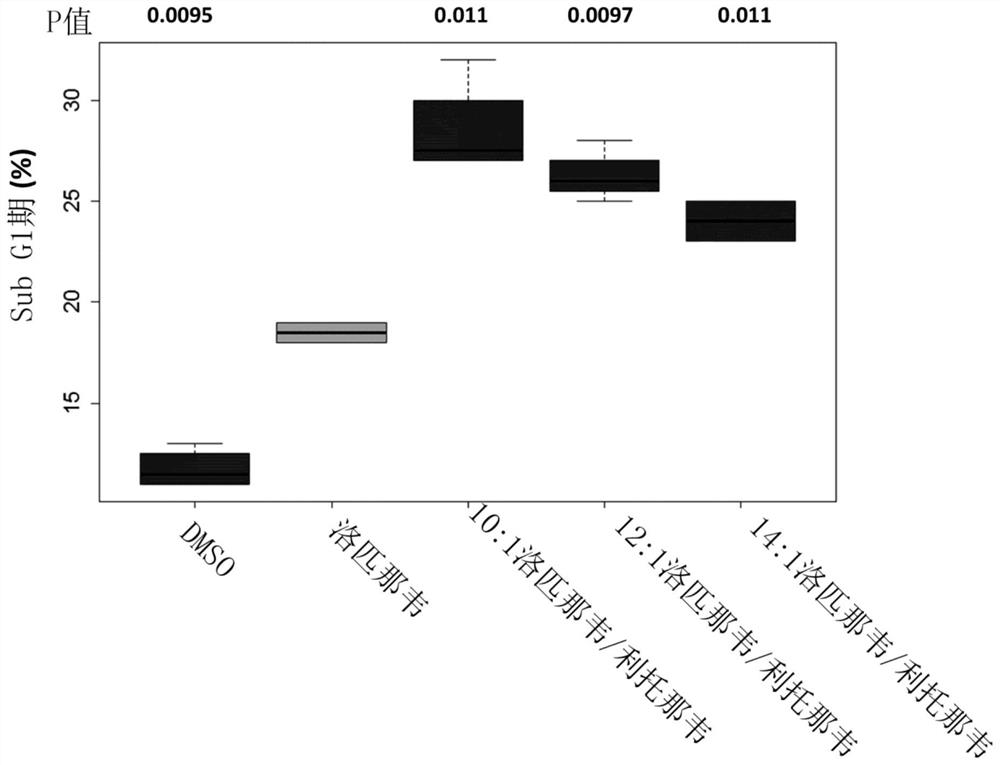
[0225] Example 2: Evaluation of the effect of lopinavir:ritonavir at a total API concentration of 25 μM in a ratio of 10:1-14:1 w / w on HaCaT cells
[0226] HaCaT cells are a spontaneously transformed but non-malignant human cell line derived from skin biopsies (Boukamp et al. (1988) J cell Biol 106(3) p761-71). The cells exhibit aneuploidy, express p53 mutations and have a phenotype comparable to AKs that progress to a cancerous lesion such as BCC or SCC. Thus, the cell line represents a good model to assess the effect of lopinavir and ritonavir on precancerous and early cancerous conditions of the skin (such as AK), as reported for the anti-apoptotic protein survivin in NMSC, Expression in AK and HaCaT cells (Grossman et al. (1999) Lab Invest 79(9) p1121-6) demonstrated.
[0227] 2.1 Method
[0228] Follow the basic method described in 1.1, except for the following:
[0229] 2.1.1 Cell culture
[0230] at 5% CO 2 HaCaT cells were cultured by standard methods using Duchen...
Embodiment 3
[0238] Example 3: Evaluation of the effect of 8:1, 12:1 and 18:1 w / w lopinavir:ritonavir at a total API concentration of 20 μM on A431 cells
[0239] A431 cells are a fully malignant epidermoid carcinoma cell line derived from a skin biopsy of an 85-year-old woman (https: / / en.wikipedia.org / wiki / A431_cells). The cells have mutant p53 and are thought to have a phenotype consistent with NMSCs, especially SCCs (Oleson (2017) Anticancer Drugs 28(10) p1106-1117). Thus, the cell line represents a good model for evaluating the effects of lopinavir and ritonavir on fully malignant NMSCs.
[0240] 3.1 Method
[0241] Follow the method described in 2.1, except for the following:
[0242] 3.1.1: A431 cells were seeded into T25 flasks and expanded in DMEM+5% FBS for 3 days, then FBS was removed for 2 days to synchronize the cells.
[0243] 3.1.2: Then add DMEM + 5% FCS and DMSO control or lopinavir alone or different ratios of lopinavir and ritonavir as described in the results section ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com