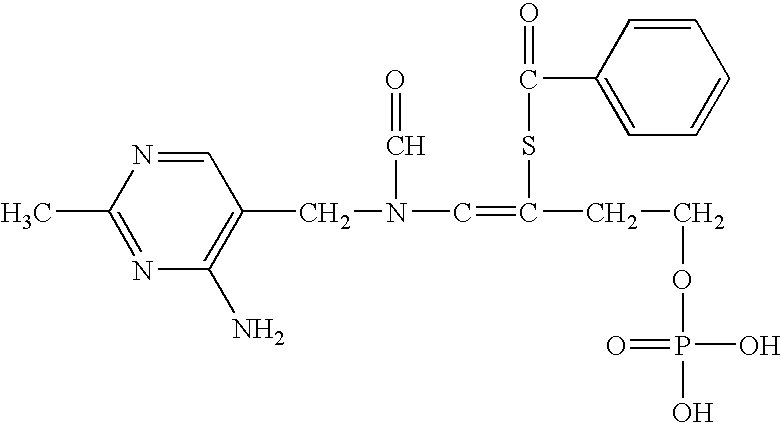
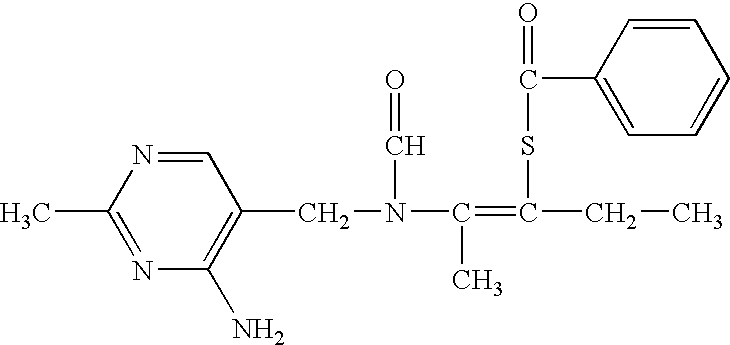
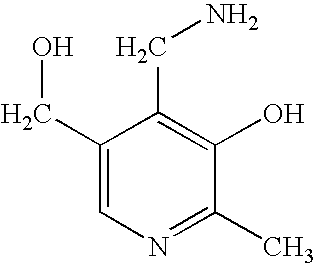
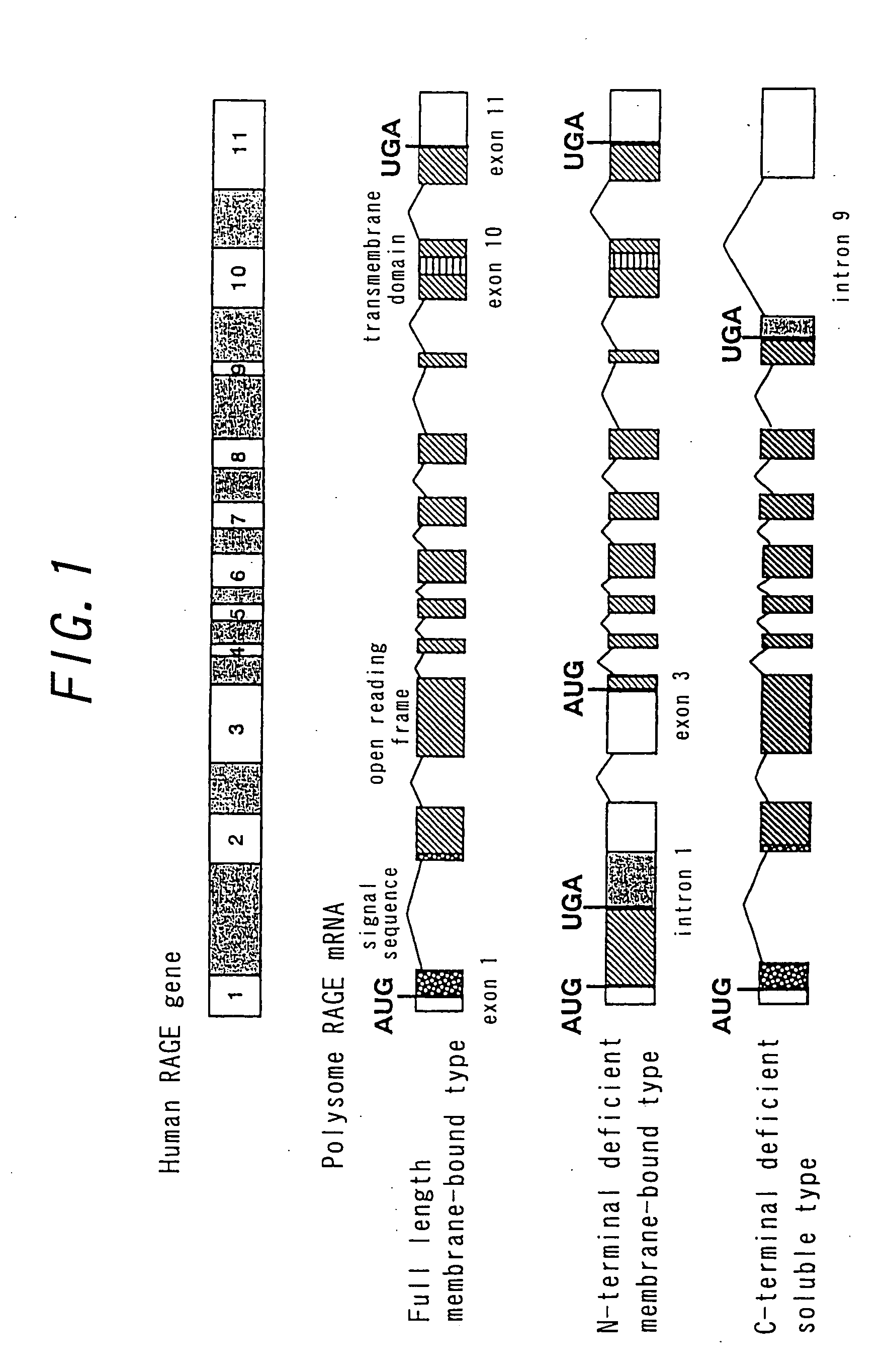
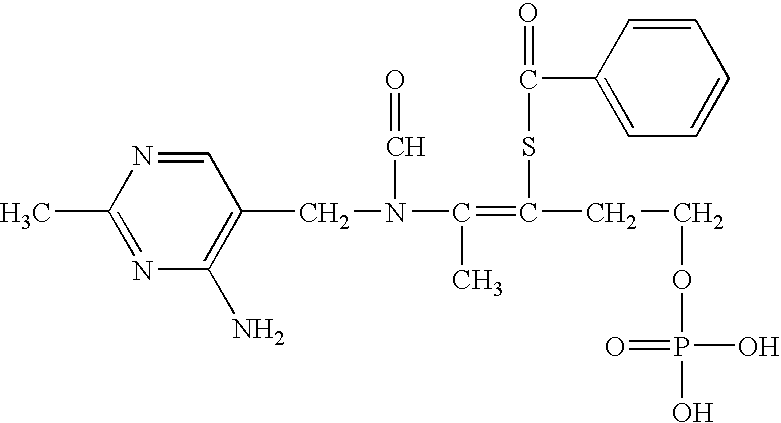
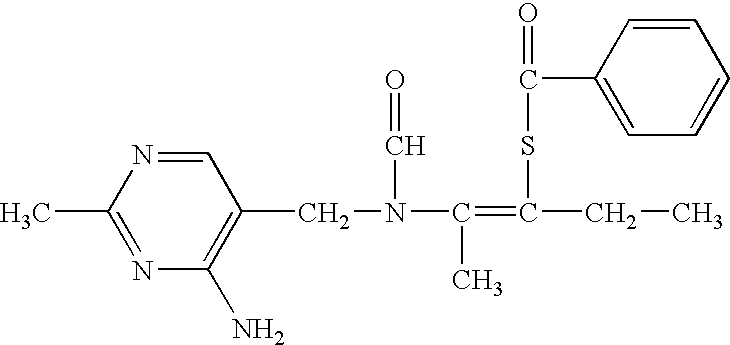
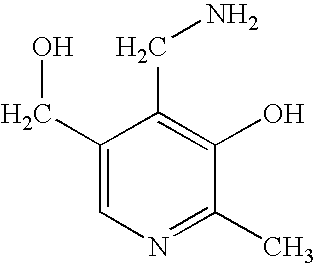
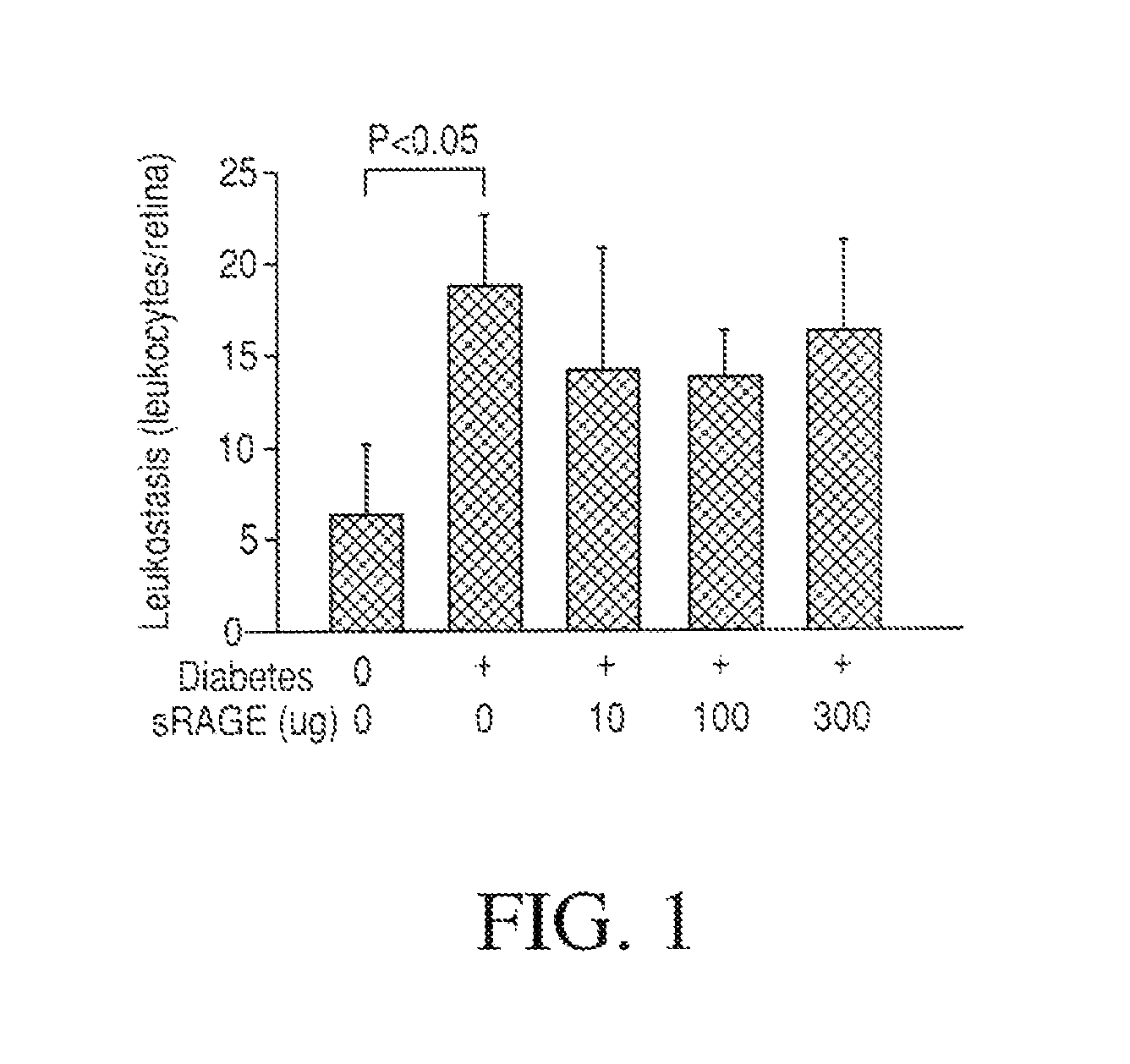
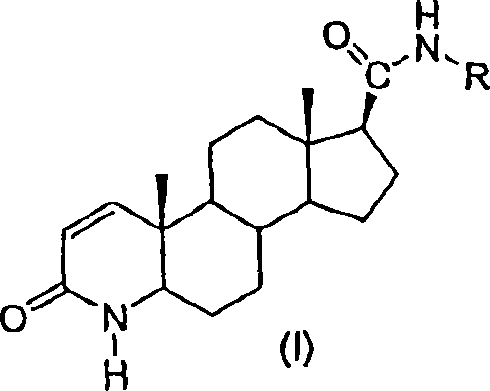
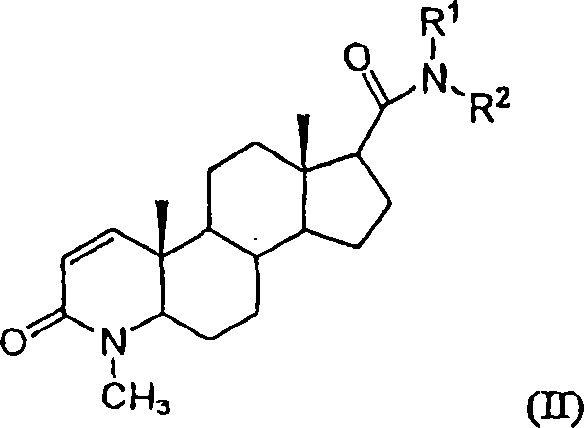
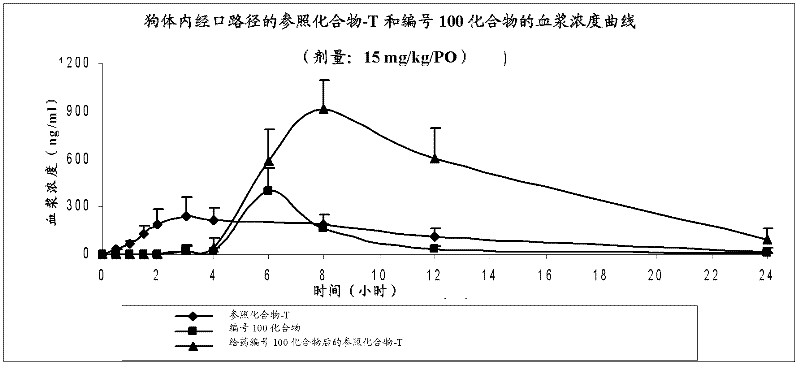
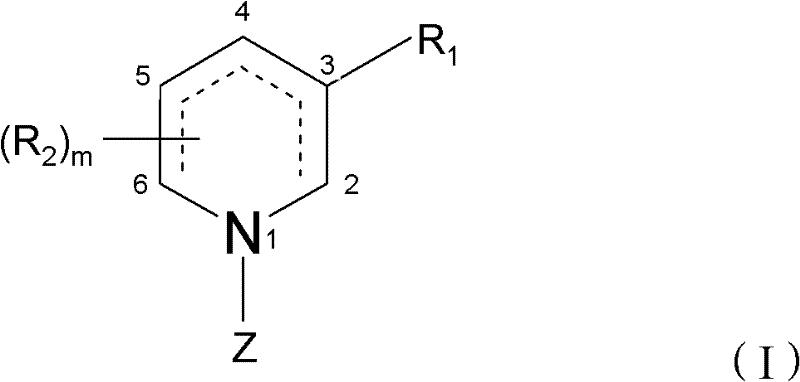
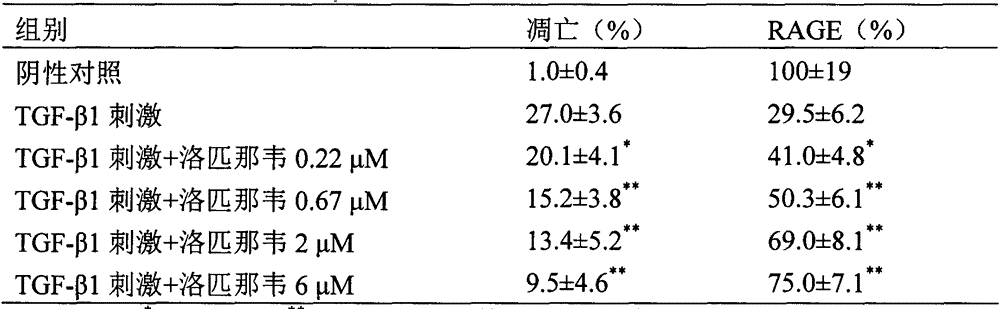
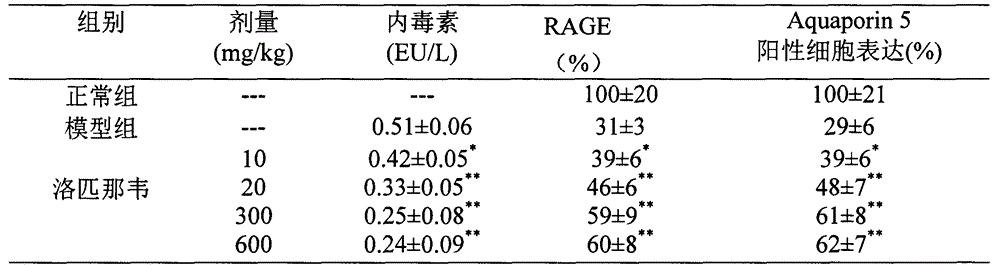
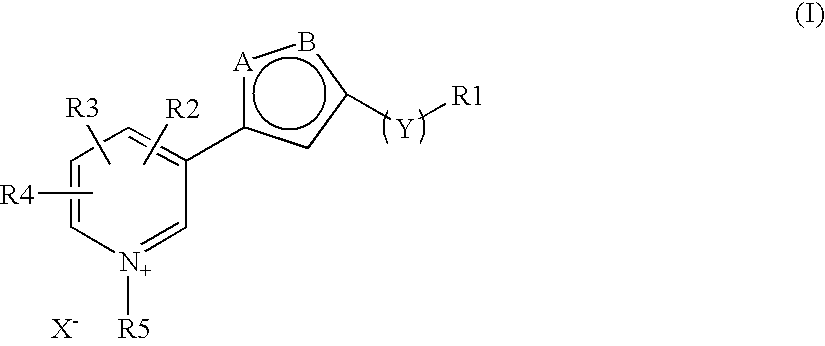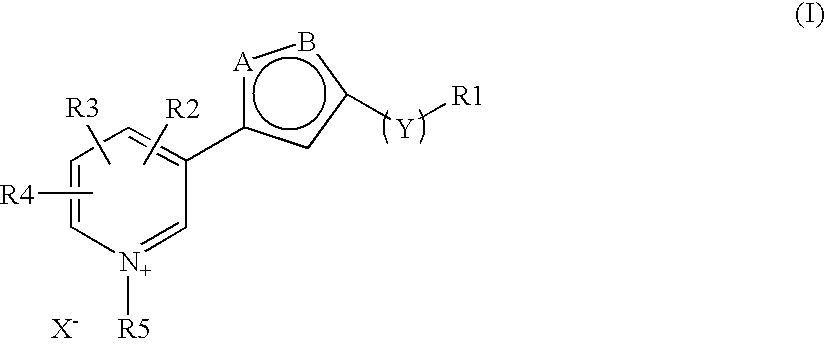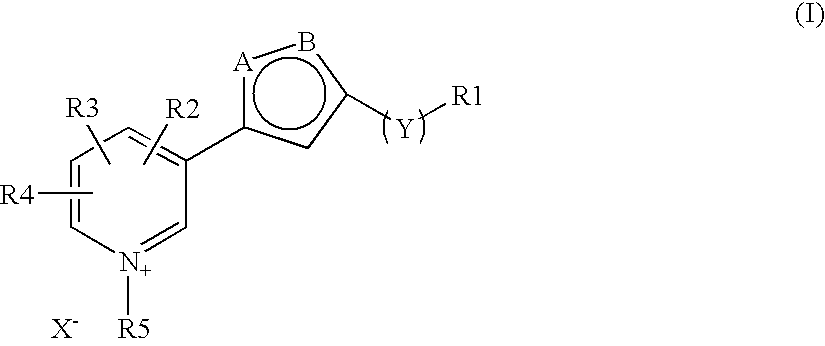Patents
Literature
45 results about "Advanced Glycation Endproducts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
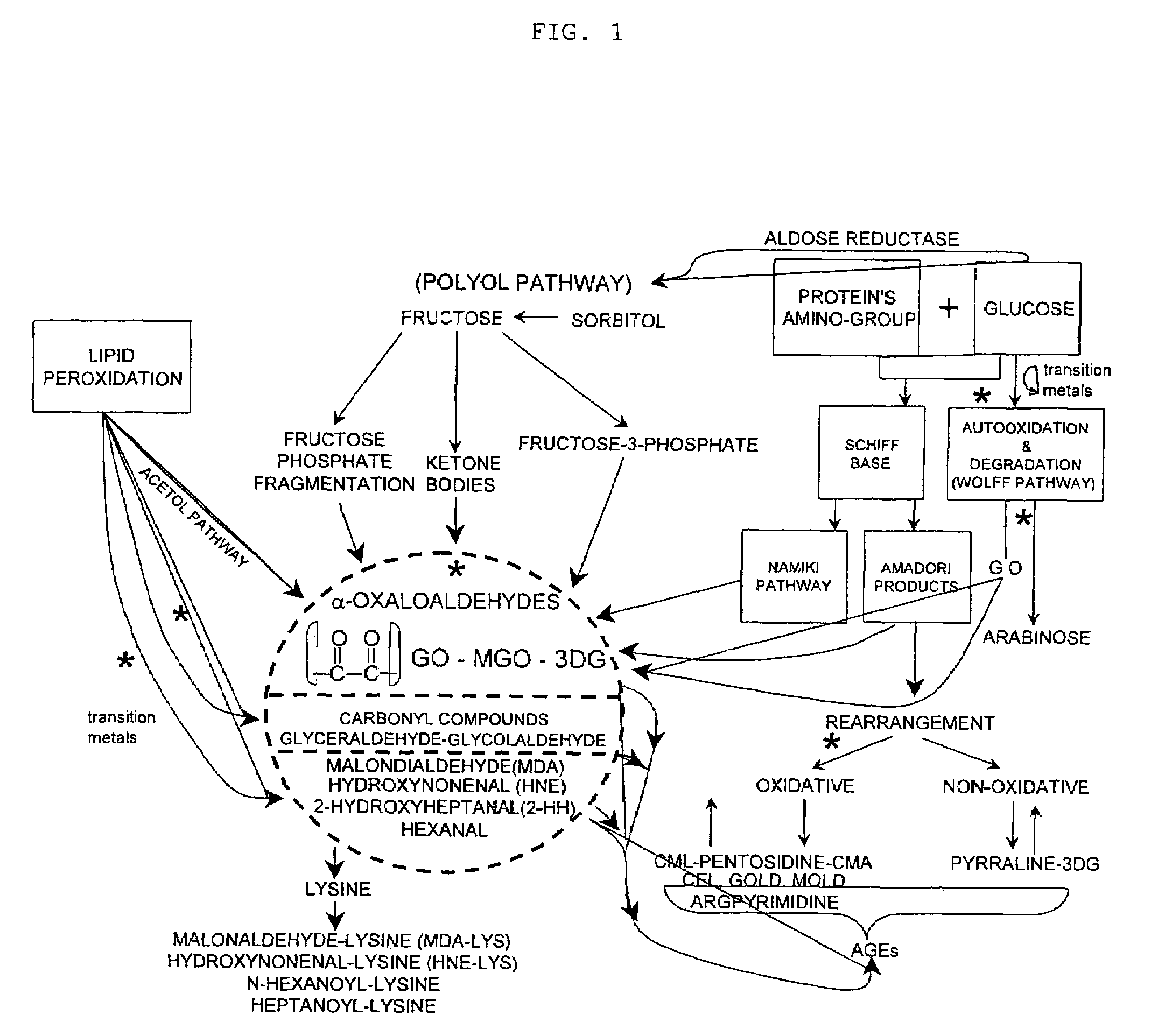
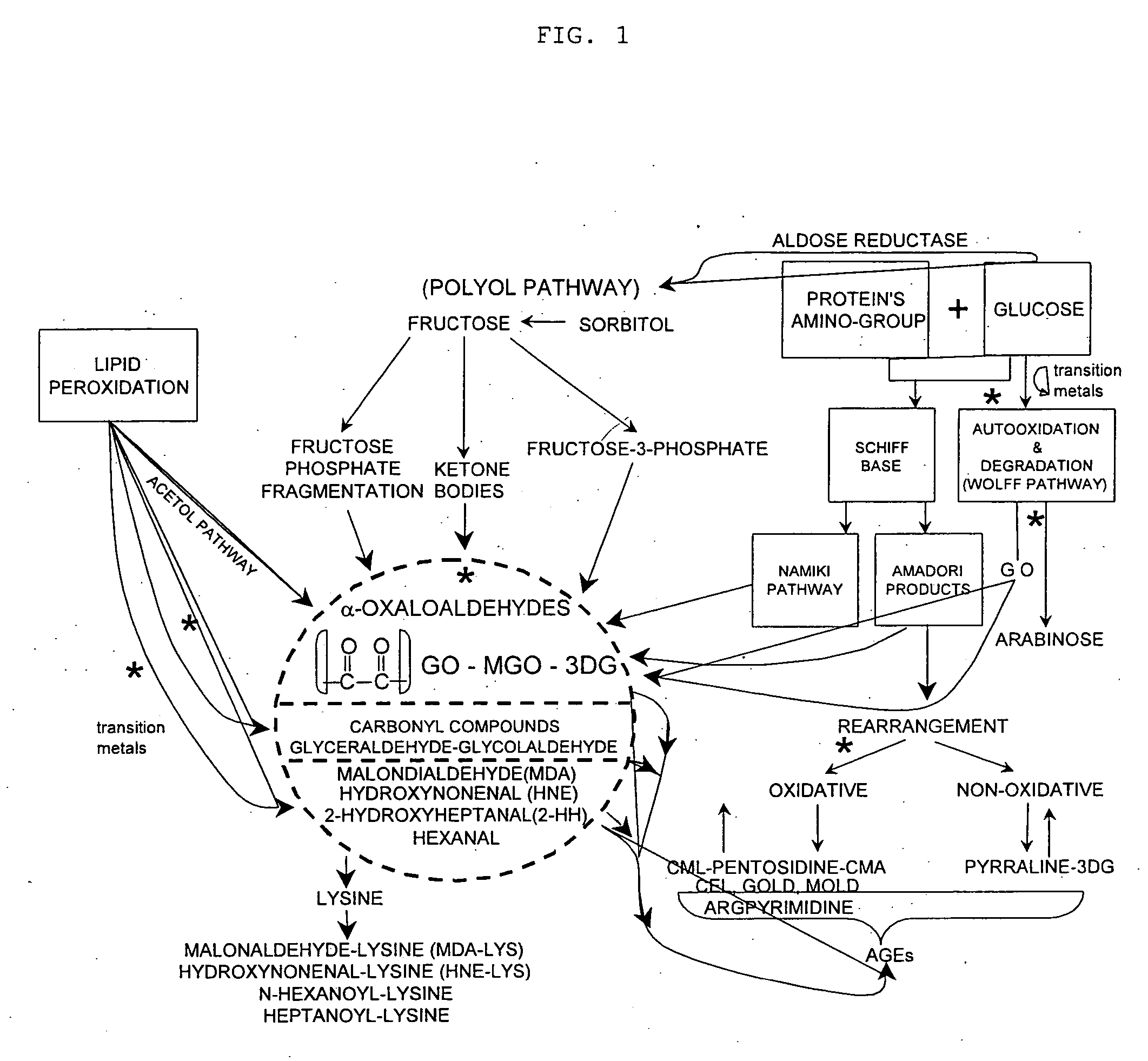
Advanced glycation end products (AGEs) are harmful compounds that are formed when protein or fat combine with sugar in the bloodstream. This process is called glycation (2).
Topical compositions comprising benfotiamine and pyridoxamine
InactiveUS20060045896A1Inhibition formationPrevention and treatment of damageBiocideOrganic active ingredientsHypopigmentationWrinkle skin
The present invention provides a composition comprising an effective amount of benfotiamine and an effective amount of pyridoxamine in a suitable vehicle for topical application. The present compositions are useful in improving the appearance of aged skin characterized by wrinkles, loss of elasticity, and hyperpigmentation caused by chronoaging and / or photoaging of skin, by inhibiting particularly skin damage resulting from reactive carbonyl species (RCS), glycation of skin proteins, formation of advanced glycation endproducts (AGEs) and formation of advanced lipoxidation endproducts (ALEs).
Owner:TRACIE MARTYN INT
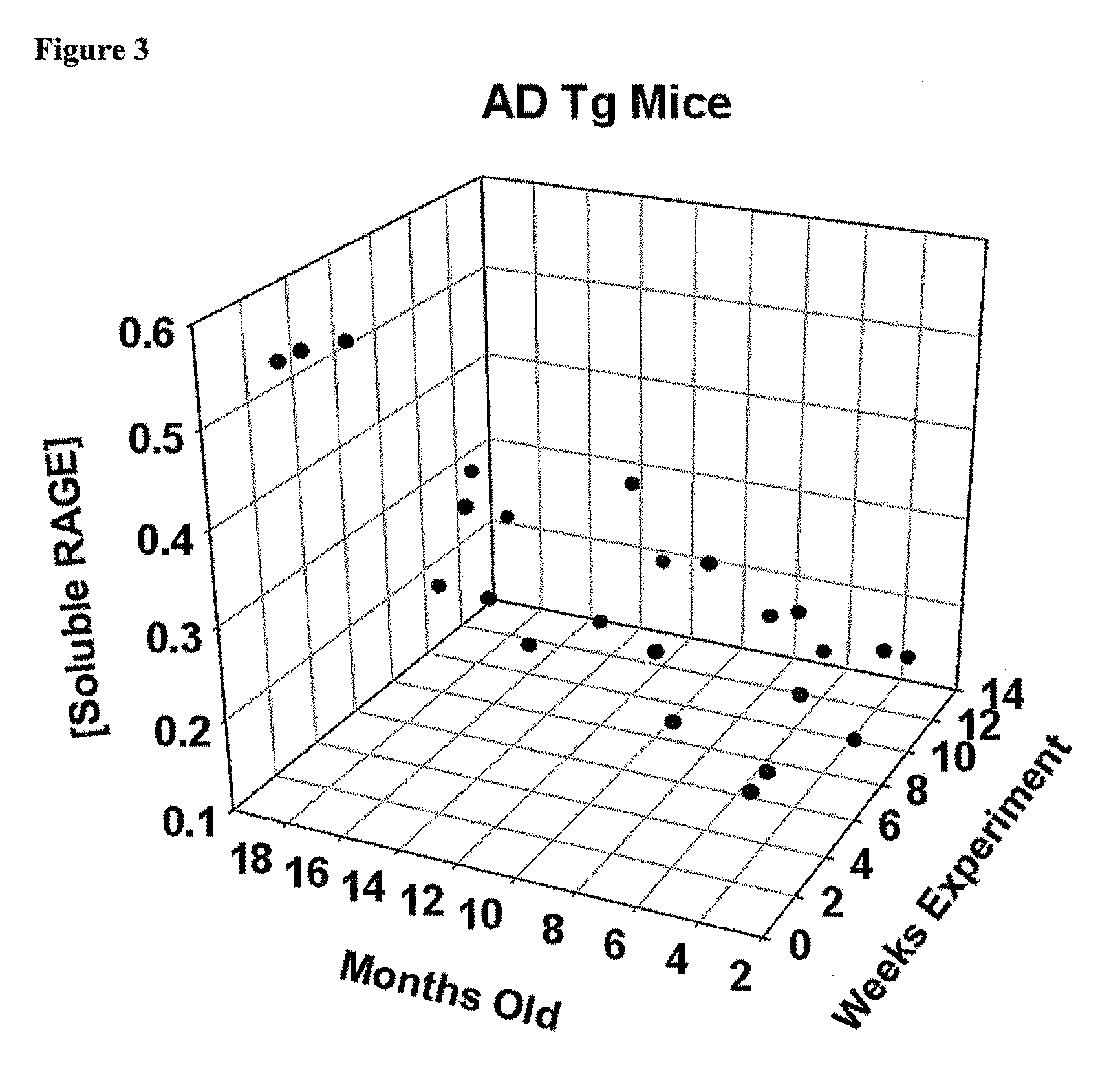
Soluble rage protein
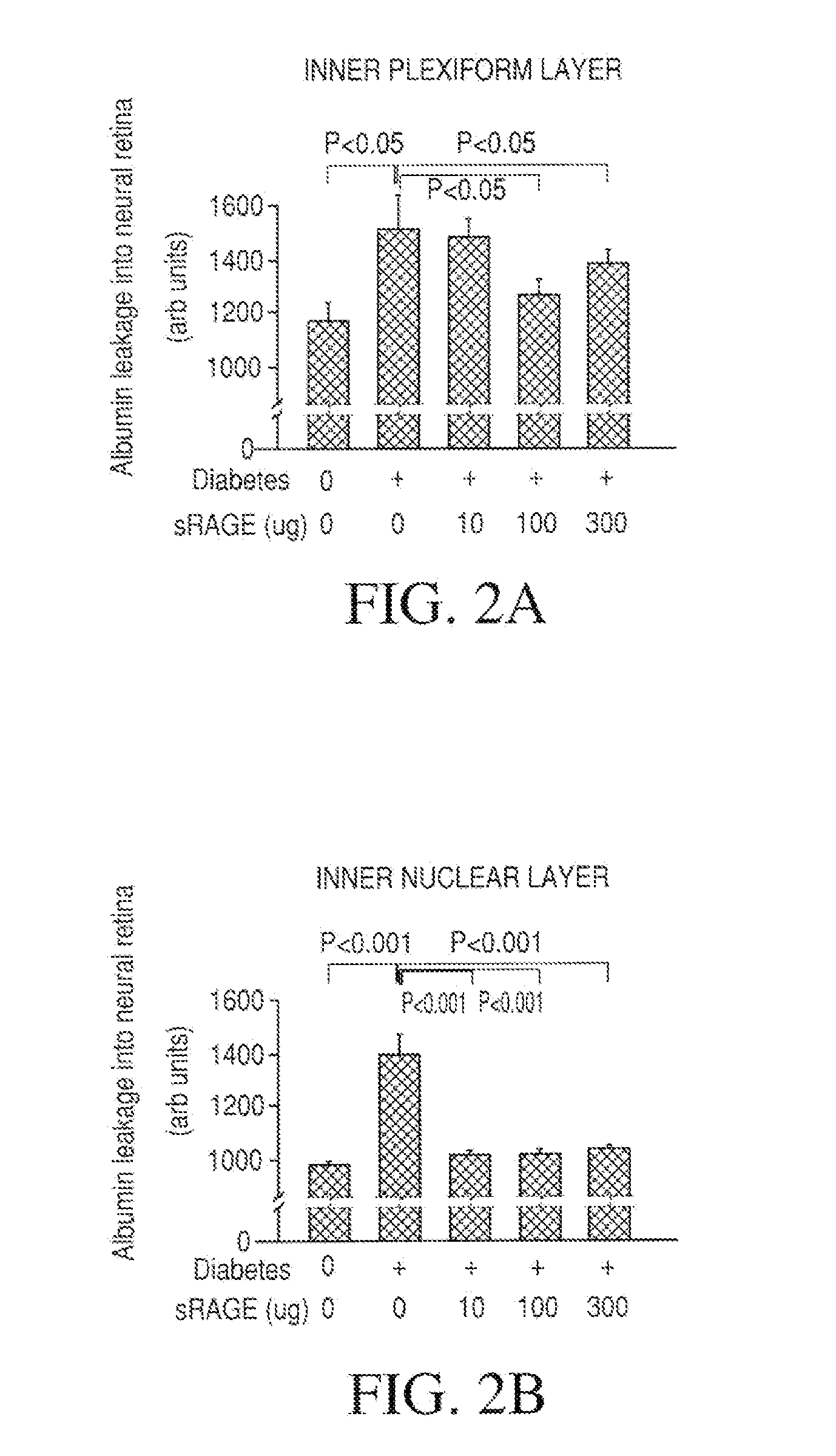
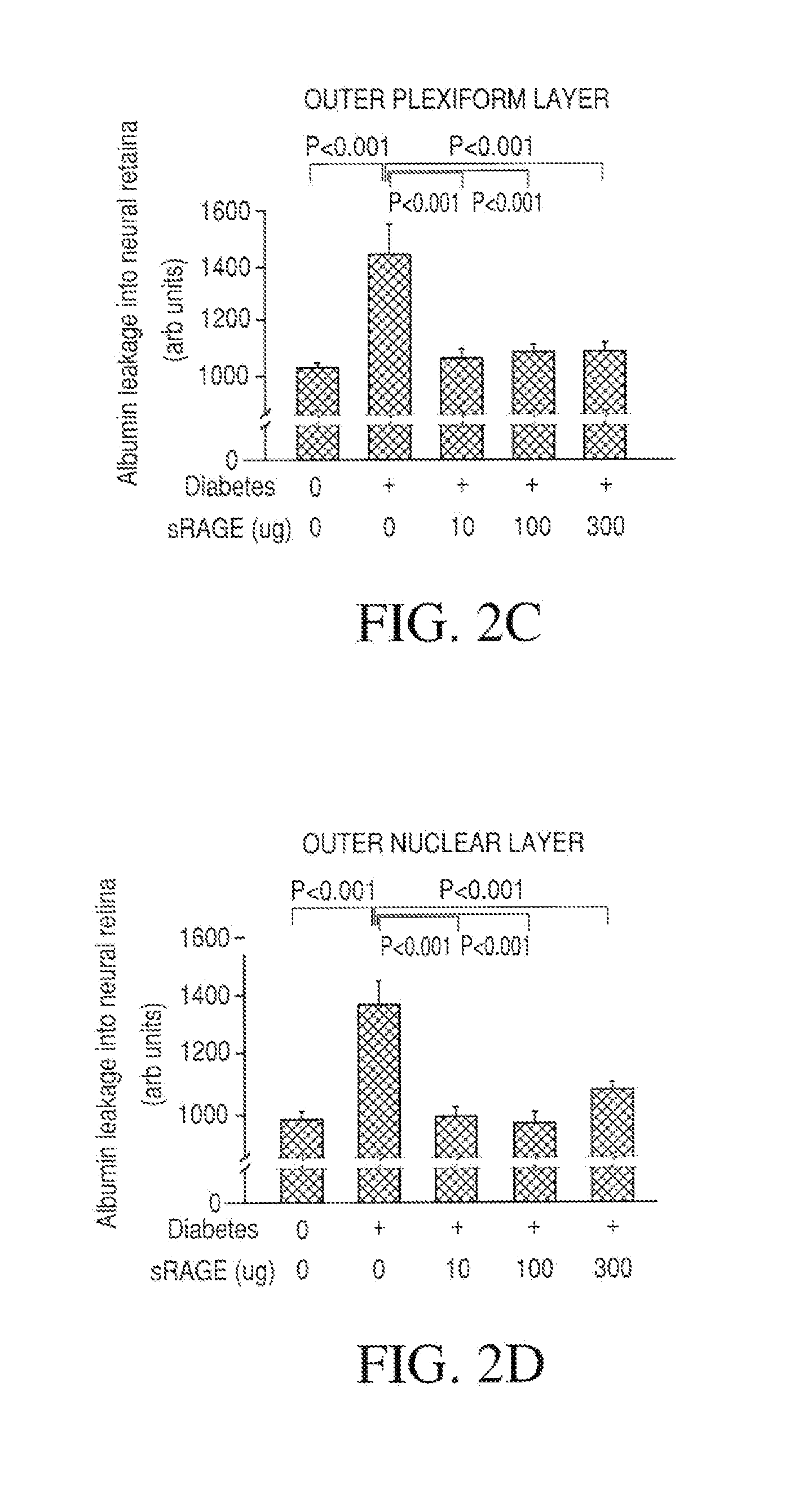
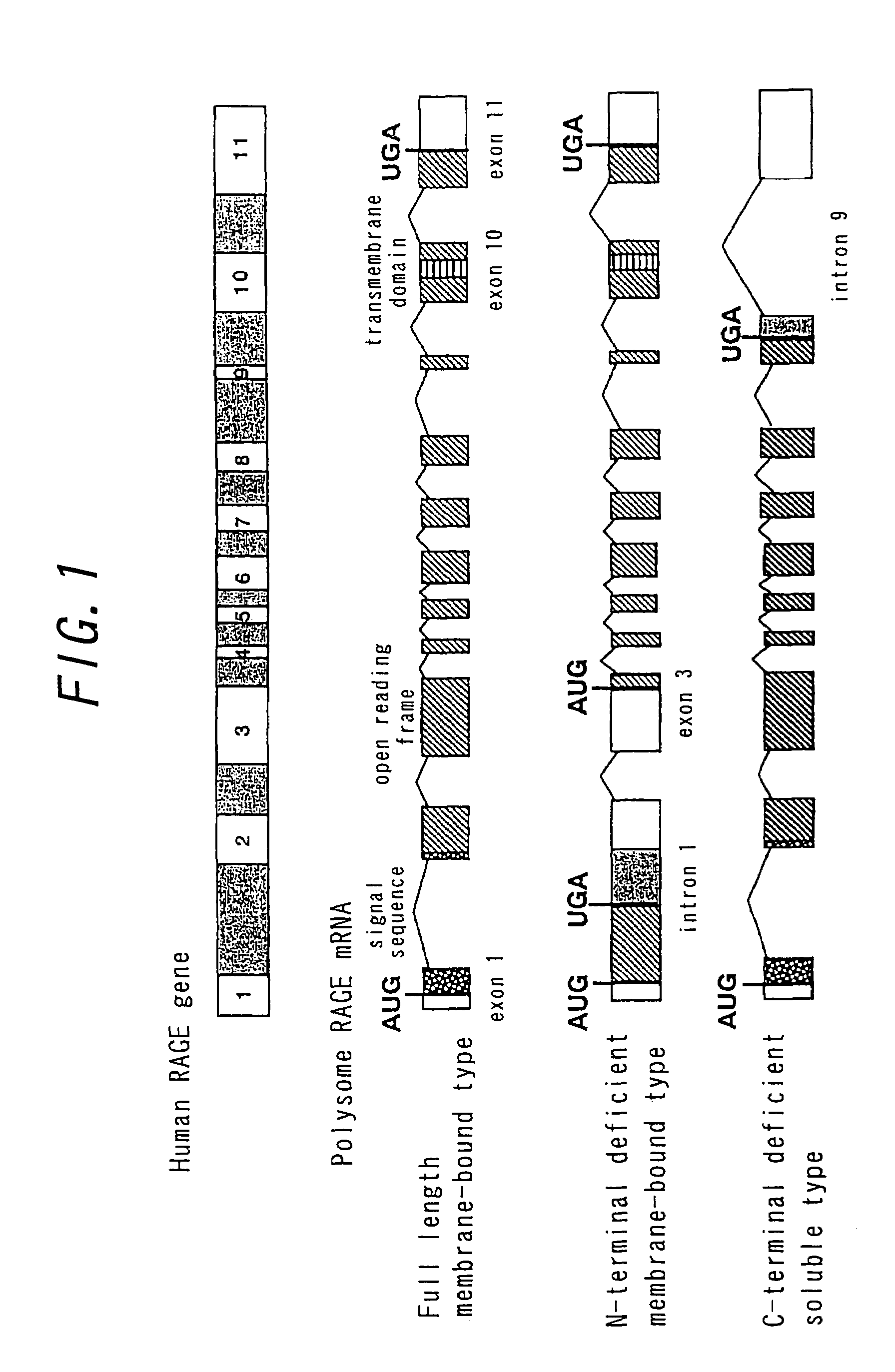
The object of this invention is to elucidate the factors involved in the regulation of AGE (advanced glycation endproducts, which are produced in a living body accompanied with diabetes and aging) and RAGE (the receptor for AGE), in order to facilitate investigation on the biological activities, physiological phenomenon and diseases related to AGE and RAGE. Plural molecular species are known to exist for RAGE, and among such molecular species, soluble RAGE exhibits the activity to modulate interaction between AGE and transmembrane-type RAGE. Using this soluble RAGE or a nucleic acid encoding it, the soluble RAGE can be measured, in addition, investigation on various physiological phenomenon, biological activities, and diseases related to interaction between AGE and RAGE can be performed to facilitate development a medicine having further efficacy.
Owner:KANAZAWA UNIV
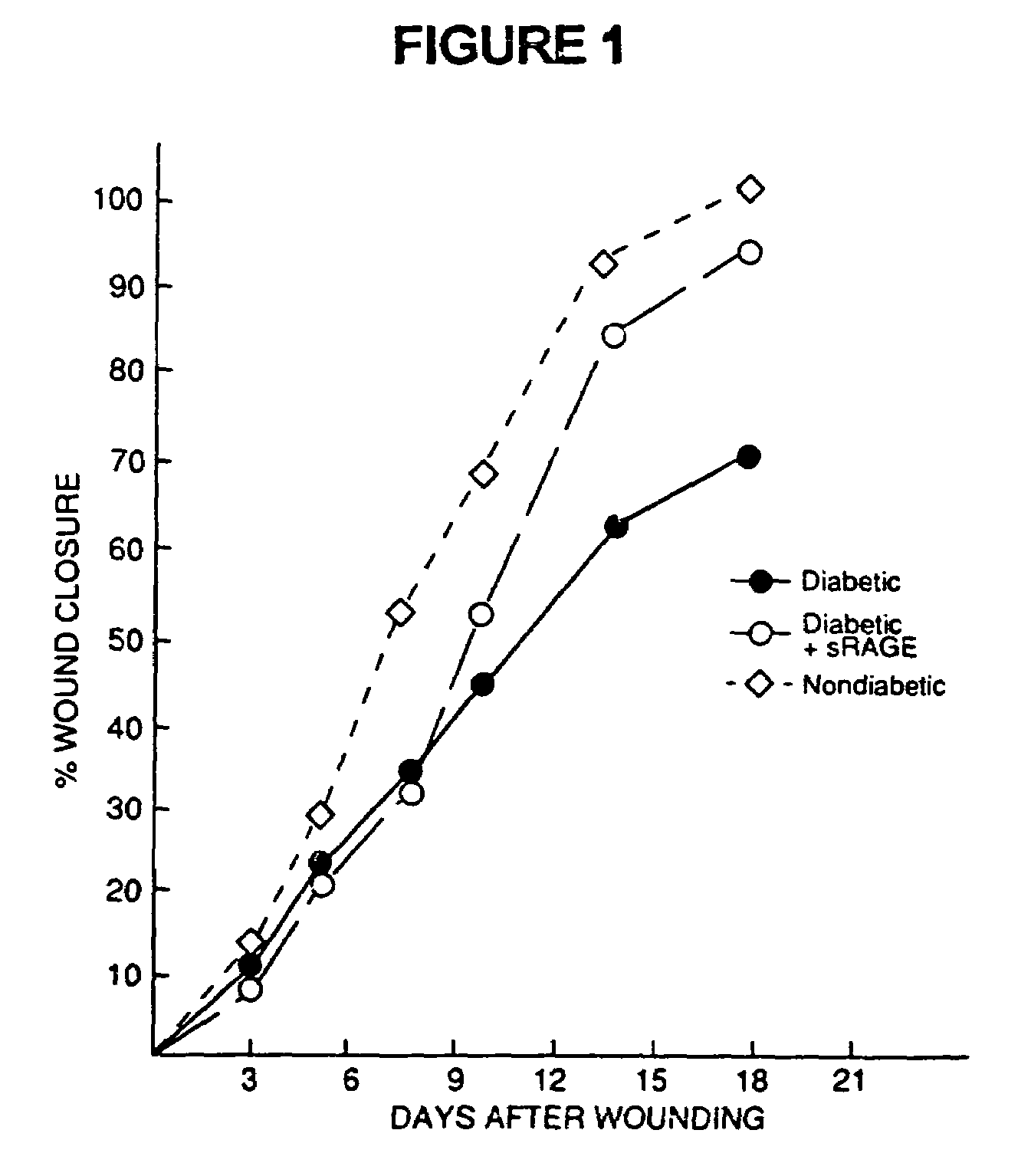
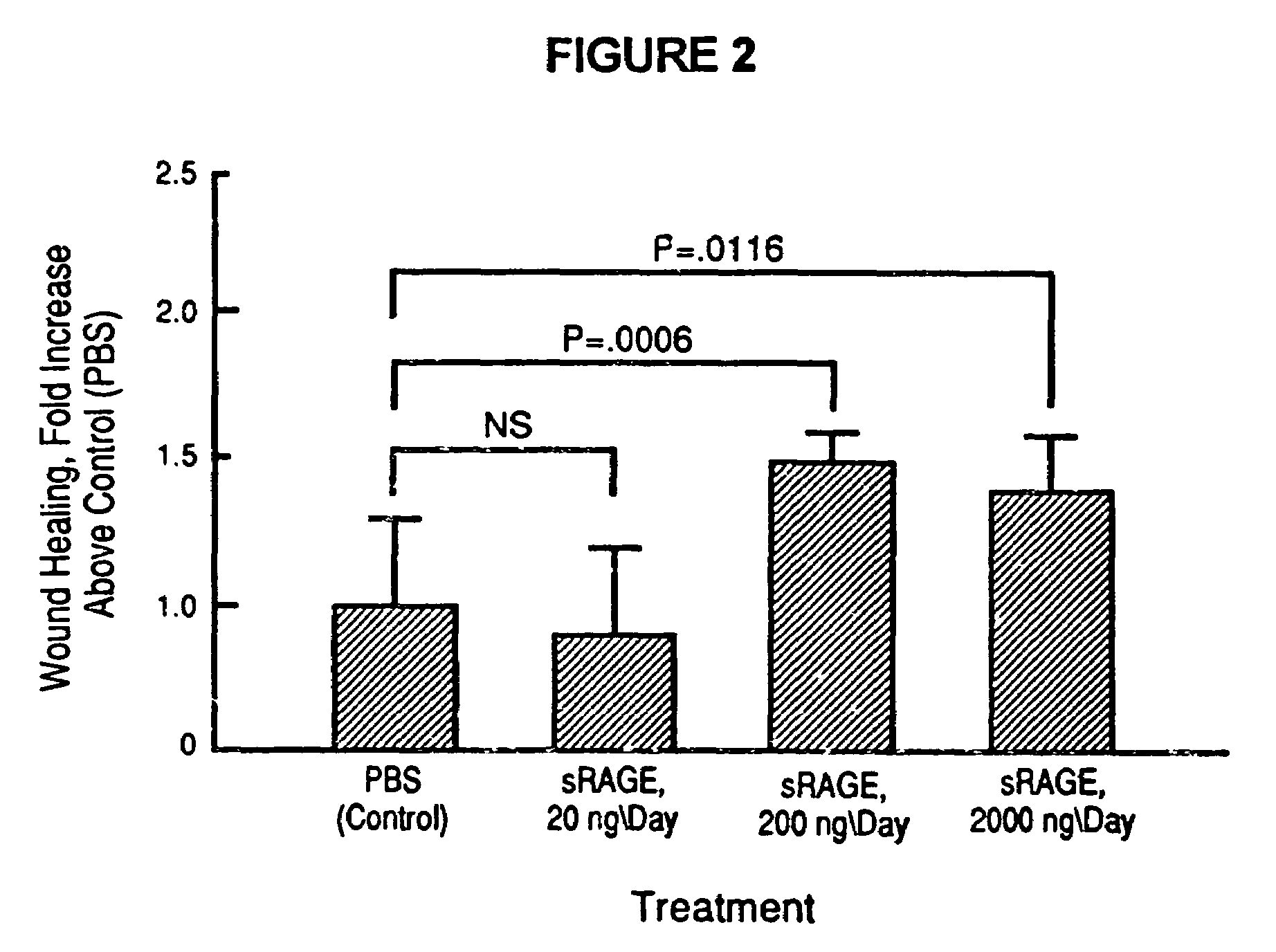
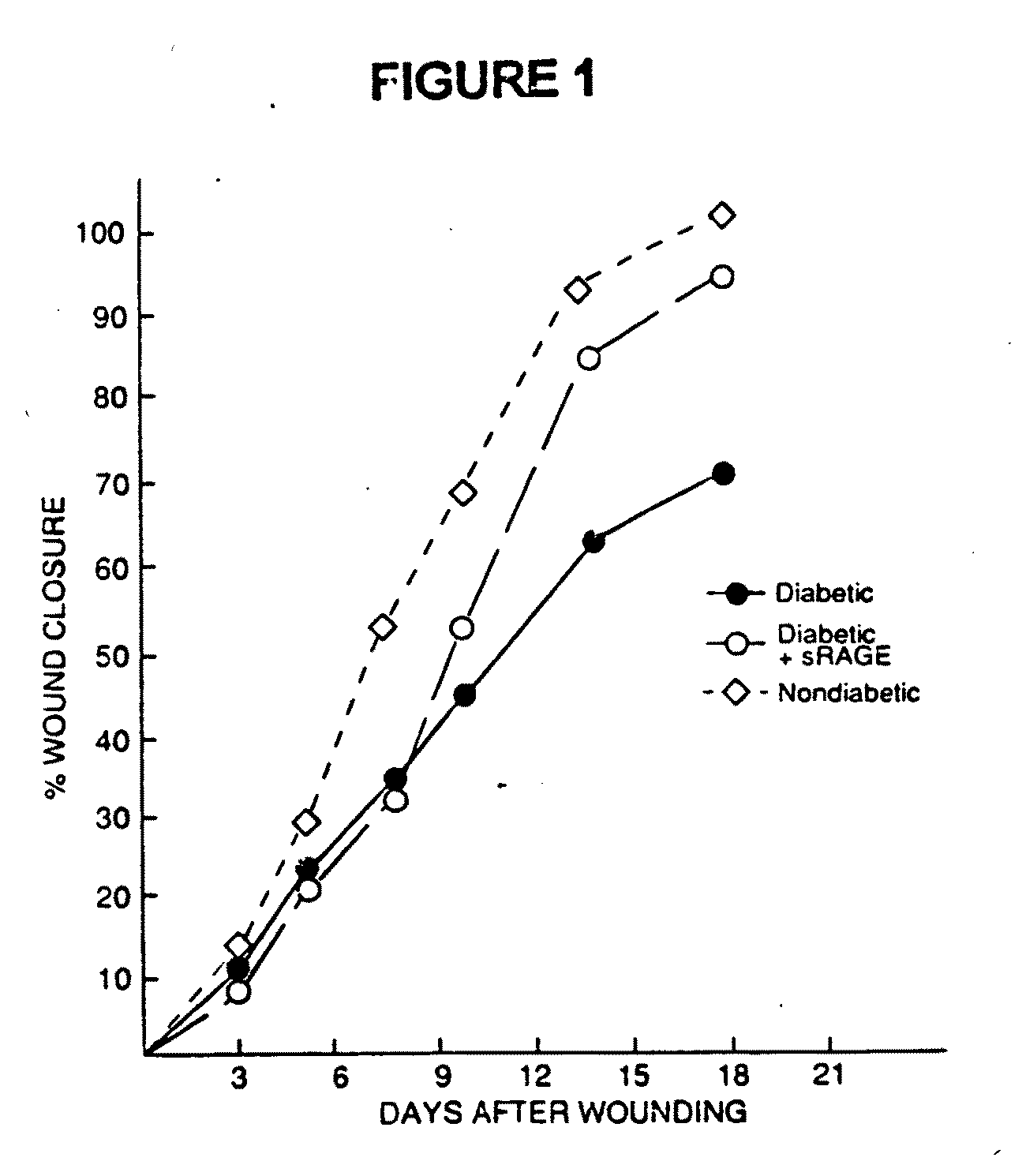
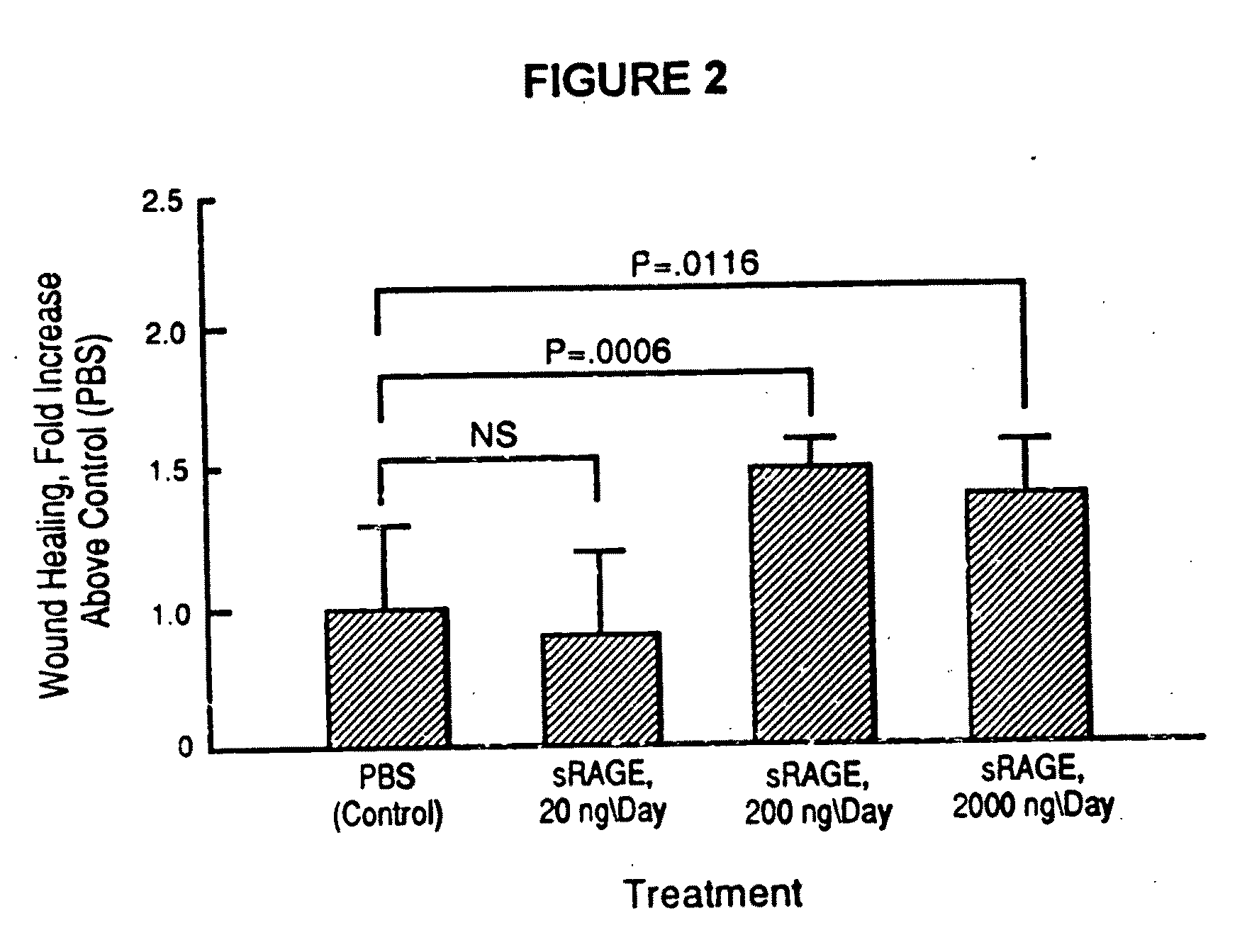
Method to prevent accelerated atherosclerosis using (sRAGE) soluble receptor for advanced glycation endproducts
InactiveUS20070099829A1Prevent accelerated atherosclerosisEffective amountPeptide/protein ingredientsMetabolism disorderMedicineMacrovascular disease
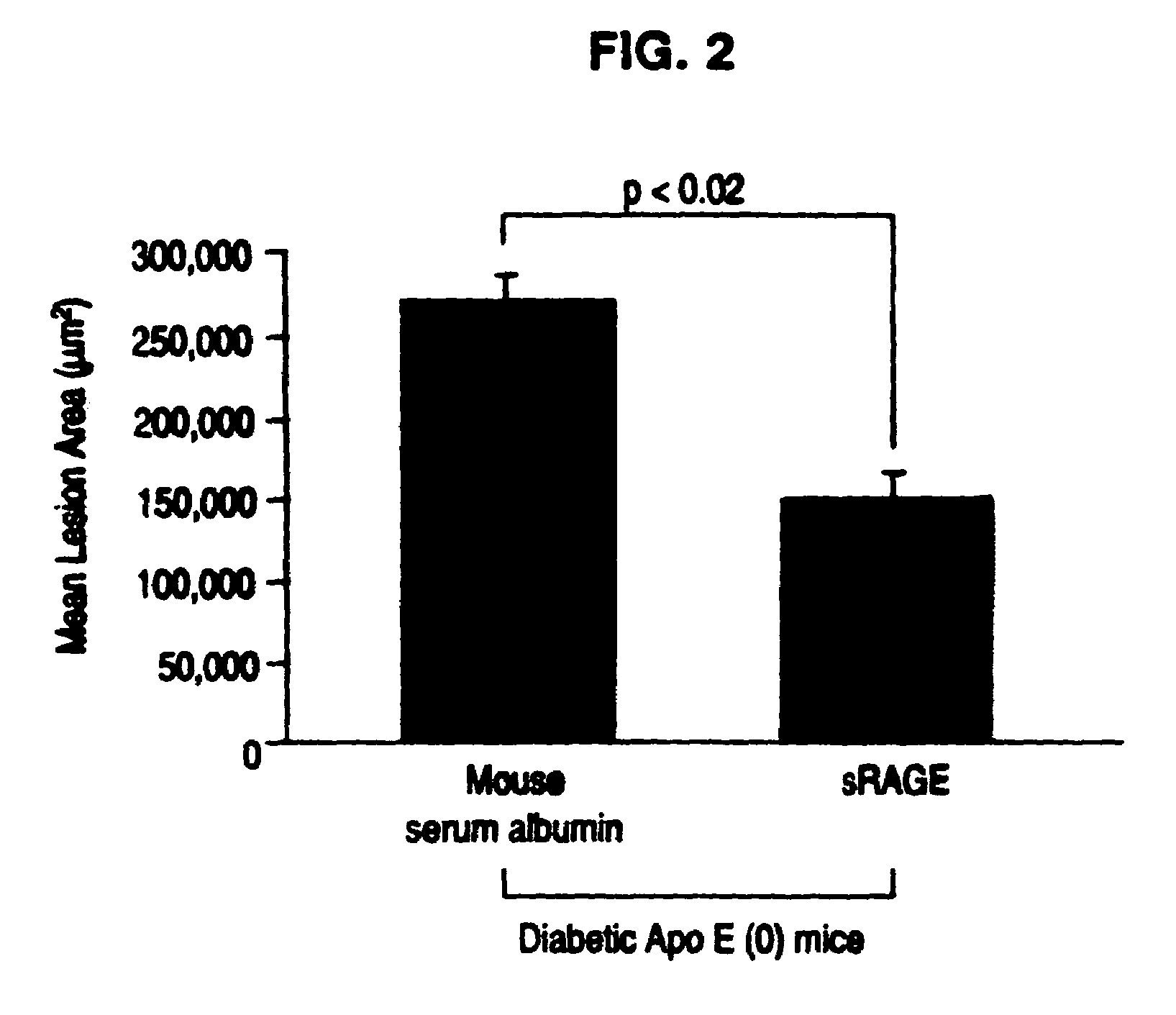
The present invention provides for a method to prevent accelerated atherosclerosis in a subject predisposed thereto which comprises administering to the subject a polypeptide derived from soluble receptor for advanced glycation endproduct in an amount effective to prevent accelerated atherosclerosis in the subject. The present invention also provides for a method to prevent a macrovessel disease in a subject predisposed thereto which comprises administering to the subject a polypeptide derived from soluble receptor for advanced glycation endproduct in an amount effective to prevent macrovessel disease in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Novel compounds for the management of aging-related and diabetic vascular complications, process for their preparation, therapeutic and cosmetic uses thereof
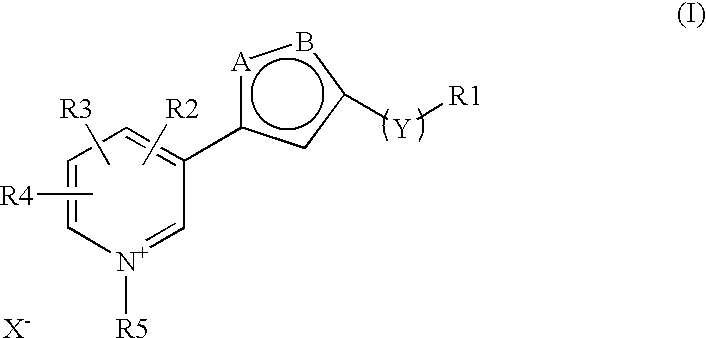
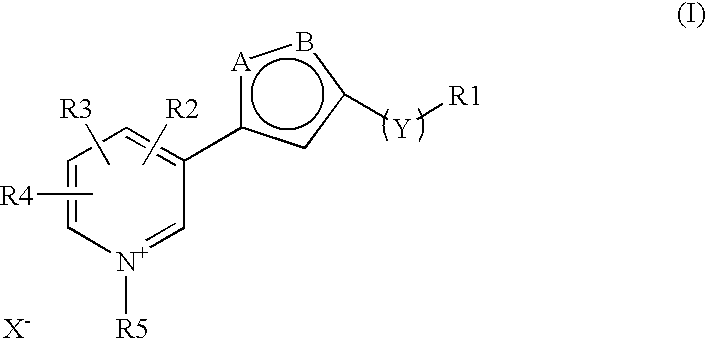
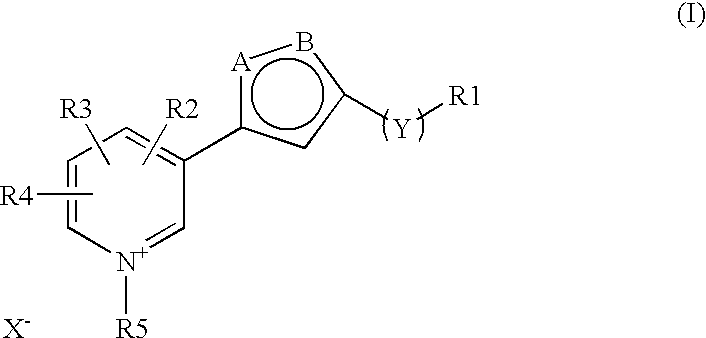
The invention discloses a new clause of a five membered heterocylic ring compounds of general formula I and its pharmaceutically or cosmetically acceptable salts, wherein R1, R2, R3, R4, R5, A, B, X and Y are as defined in the specification. The invention also discloses a process for preparation of these compound and their therapeutic and cosmetic applications particularly in the management of aging related and diabetic vascular complications. The compounds in question act by triple action of an AGE (Advanced Glycation Endproducts) breaker, AGE inhibitor and free radical scavenger which make them most suitable in different therapeutic and cosmetic applications. The invention also discloses pharmaceuticals and cosmetic compositions comprising these compounds and method of treatment of diseases caused by accumulation of AGE and / or free radicals in the body cells.
Owner:TORRENT PHARMA LTD
Method for inhibiting new tissue growth in blood vessels in a patient subjected to blood vessel injury
InactiveUS20080171701A1Preventing exaggerated restenosisPrevent exaggerated restenosisOrganic active ingredientsPeptide/protein ingredientsBlood vessel spasmPercent Diameter Stenosis
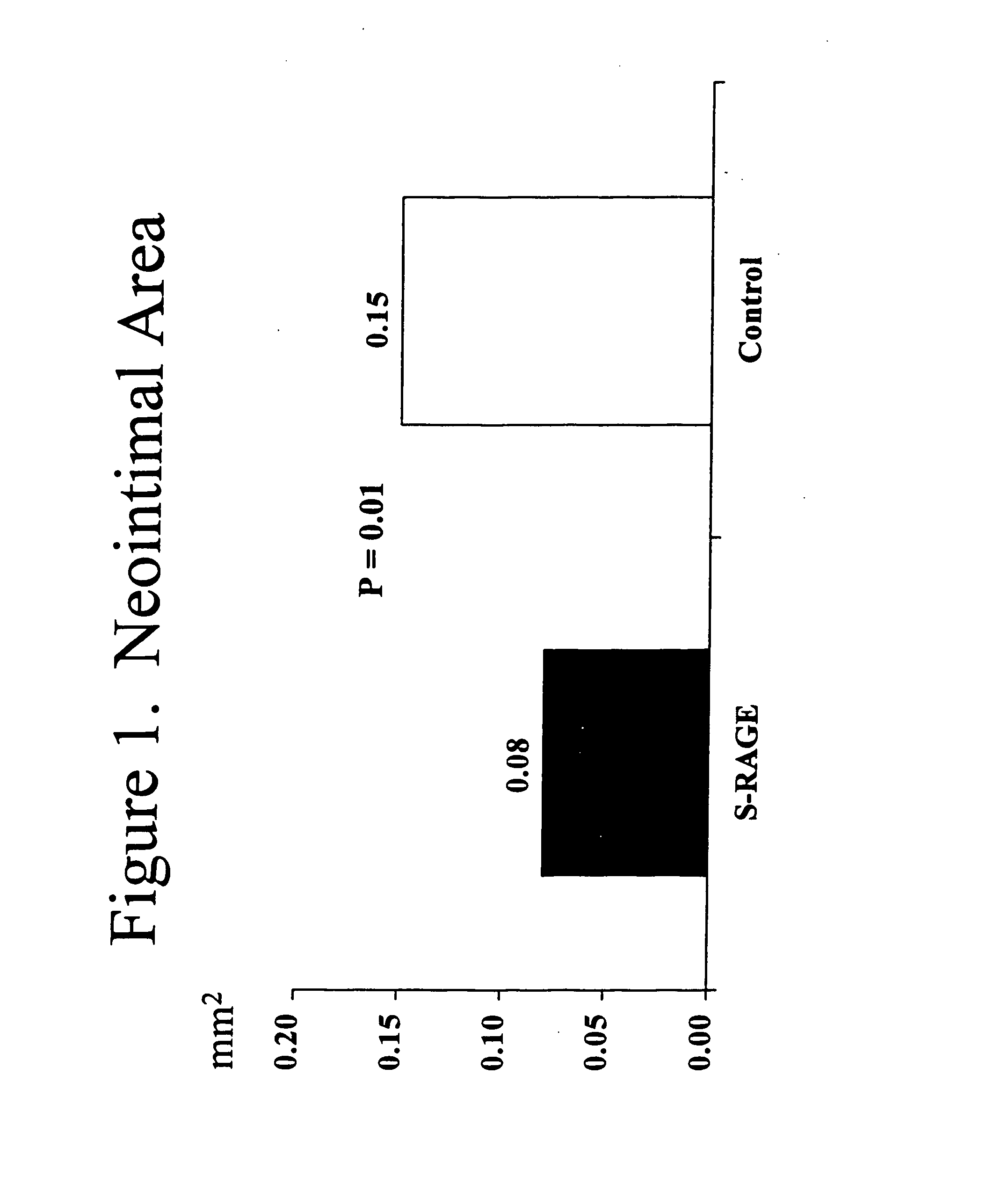
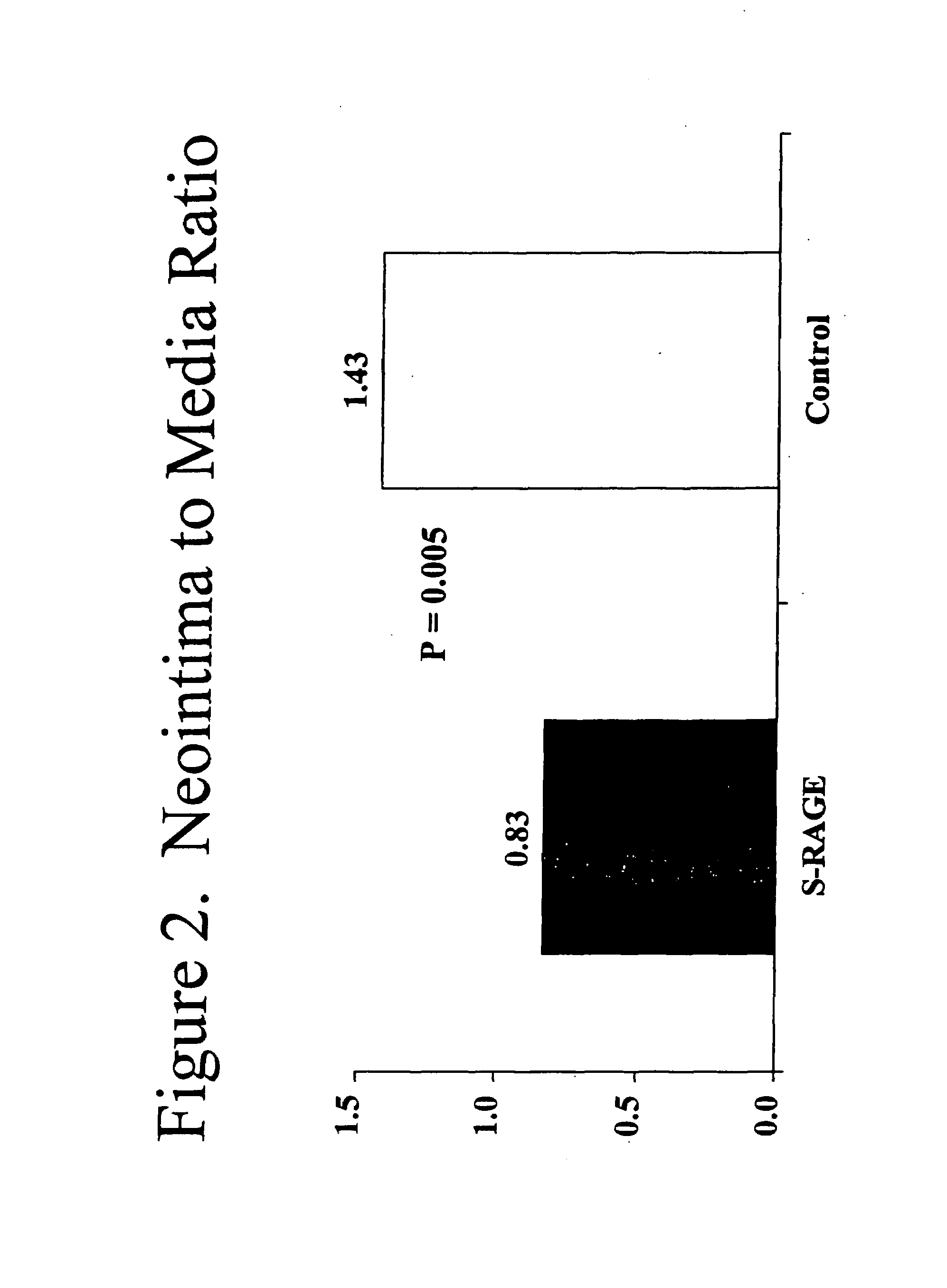
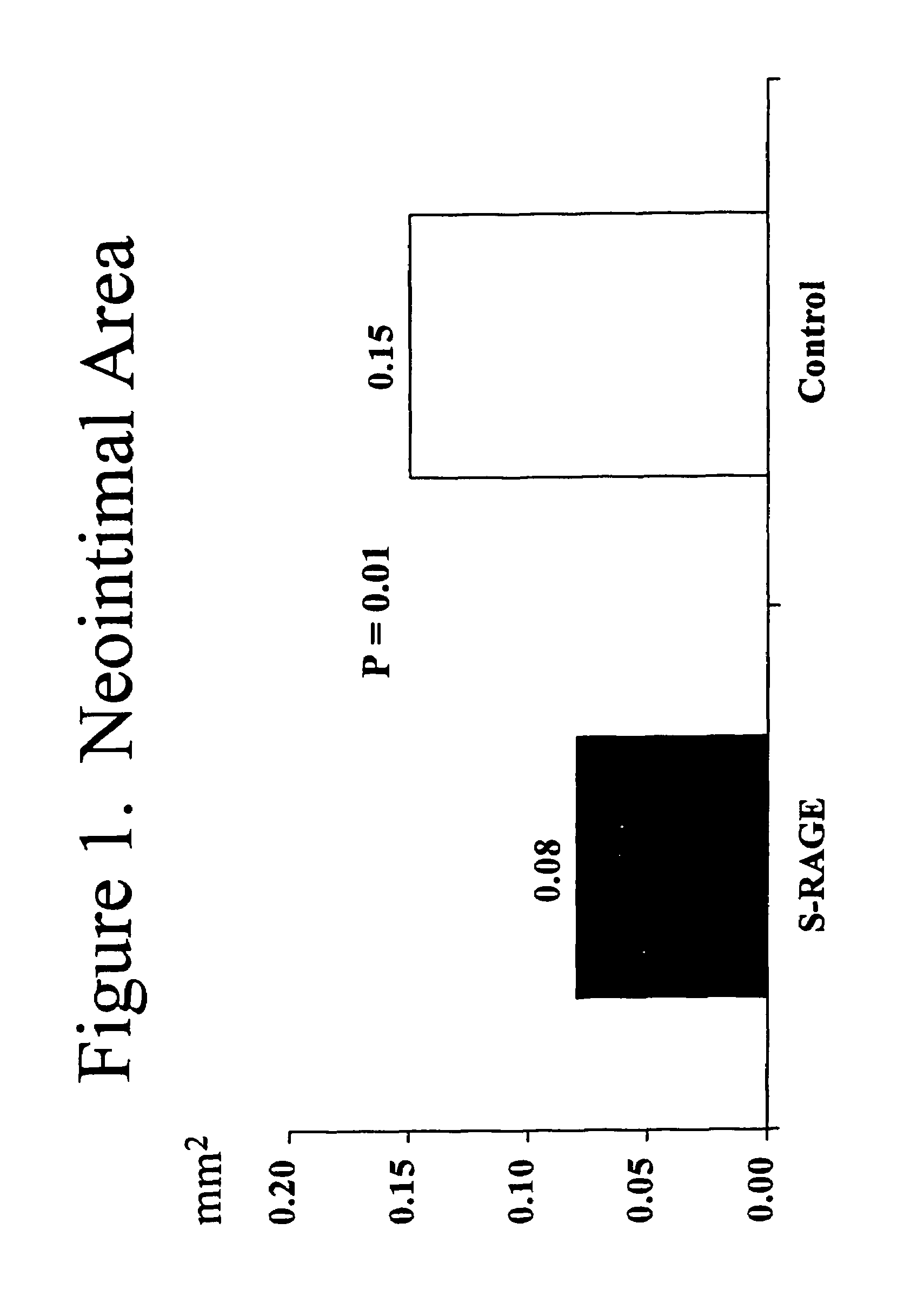
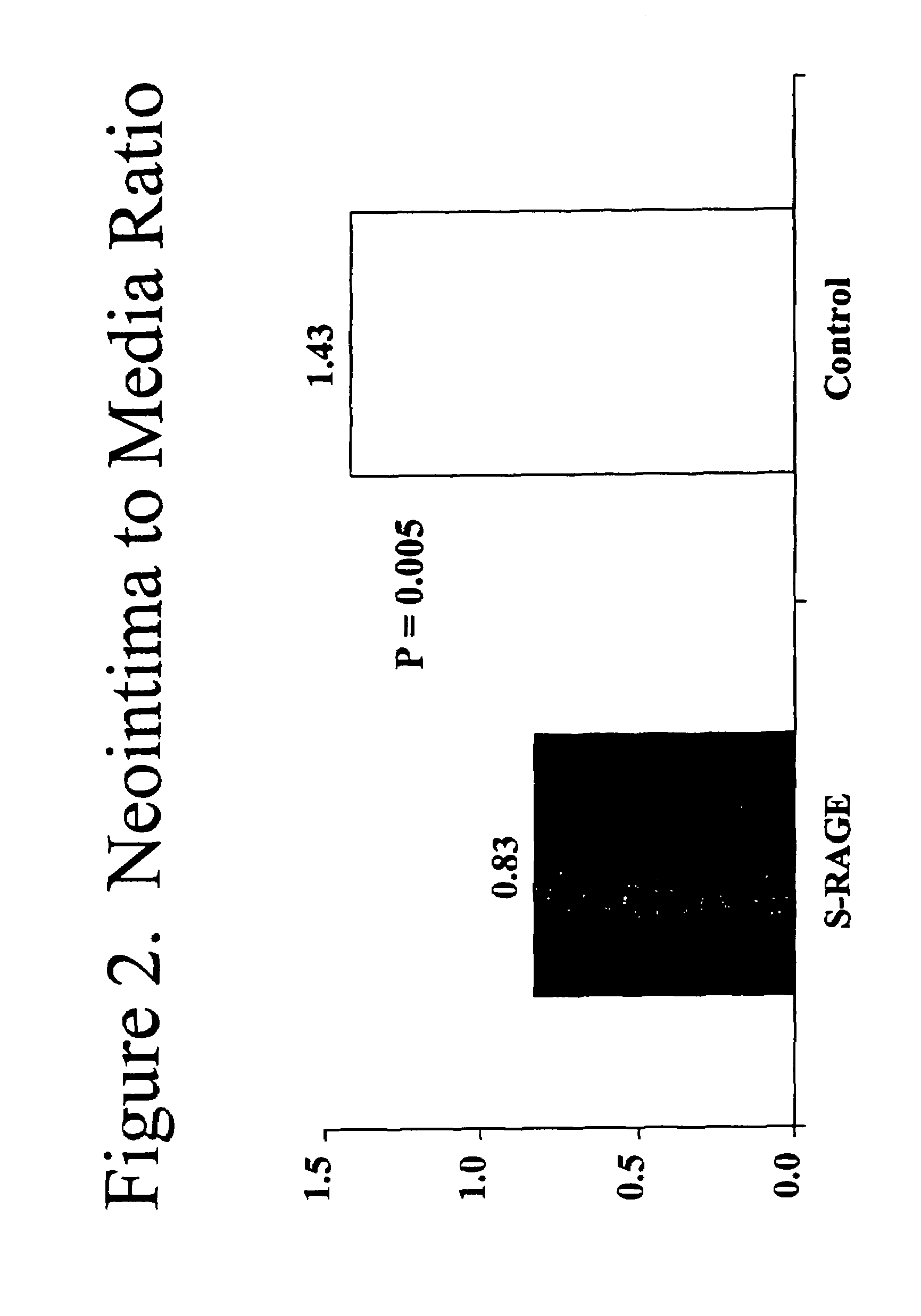
This invention provides for a method for inhibiting new tissue growth in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit new tissue growth in the subject's blood vessels. The invention also provides for method for inhibiting neointimal formation in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit neointimal formation in the subject's blood vessels. The invention also provides a method for preventing exaggerated restenosis in a diabetic subject which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to prevent exaggerated restenosis in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Topical compositions comprising benfotiamine and pyridoxamine
The present invention provides a composition comprising an effective amount of benfotiamine and an effective amount of pyridoxamine in a suitable vehicle for topical application. The present compositions are useful in improving the appearance of aged skin characterized by wrinkles, loss of elasticity, and hyperpigmentation caused by chronoaging and / or photoaging of skin, by inhibiting particularly skin damage resulting from reactive carbonyl species (RCS), glycation of skin proteins, formation of advanced glycation endproducts (AGEs) and formation of advanced lipoxidation endproducts (ALEs).
Owner:TRACIE MARTYN INT
Rage fusion proteins
The present invention provides novel therapeutics and methods of treatment for diseases associated with activation of the advanced glycation endproducts receptor (RAGE).
Owner:GALACTICA PHARMA
Soluble rage protein
The object of this invention is to elucidate the factors involved in the regulation of AGE (advanced glycation endproducts, which are produced in a living body accompanied with diabetes and aging) and RAGE (the receptor for AGE), in order to facilitate investigation on the biological activities, physiological phenomenon and diseases related to AGE and RAGE. Plural molecular species are known to exist for RAGE, and among such molecular species, soluble RAGE exhibits the activity to modulate interaction between AGE and transmembrane-type RAGE. Using this soluble RAGE or a nucleic acid encoding it, the soluble RAGE can be measured, in addition, investigation on various physiological phenomenon, biological activities, and diseases related to interaction between AGE and RAGE can be performed to facilitate development a medicine having further efficacy.
Owner:KANAZAWA UNIV
Method for inhibiting new tissue growth in blood vessels in a patient subjected to blood vessel injury
InactiveUS7732400B2Preventing exaggerated restenosisPrevent exaggerated restenosisBiocideOrganic active ingredientsDiabetes mellitusPercent Diameter Stenosis
This invention provides for a method for inhibiting new tissue growth in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit new tissue growth in the subject's blood vessels. The invention also provides for method for inhibiting neointimal formation in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit neointimal formation in the subject's blood vessels. The invention also provides a method for preventing exaggerated restenosis in a diabetic subject which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to prevent exaggerated restenosis in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Method for inhibiting accelerated atherosclerosis in a subject suffering from hypercholesterolemia or hypertriglyceridemia
InactiveUS7494972B2Prevent accelerated atherosclerosisPrevent macrovessel diseasePeptide/protein ingredientsMetabolism disorderHypertriglyceridemiaMedicine
The present invention provides for a method to prevent accelerated atherosclerosis in a subject predisposed thereto which comprises administering to the subject a polypeptide derived from soluble receptor for advanced glycation endproduct in an amount effective to prevent accelerated atherosclerosis in the subject. The present invention also provides for a method to prevent a macrovessel disease in a subject predisposed thereto which comprises administering to the subject a polypeptide derived from soluble receptor for advanced glycation endproduct in an amount effective to prevent macrovessel disease in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
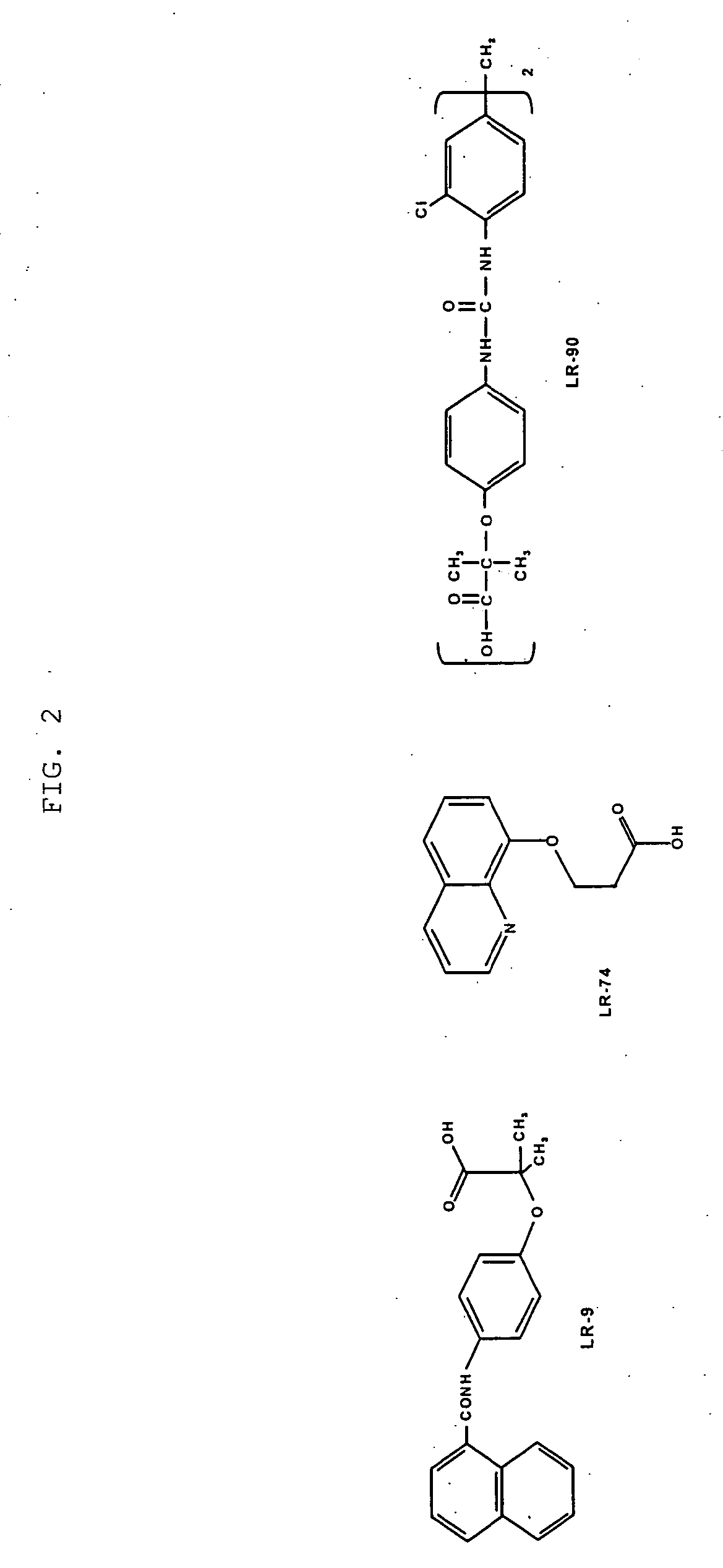
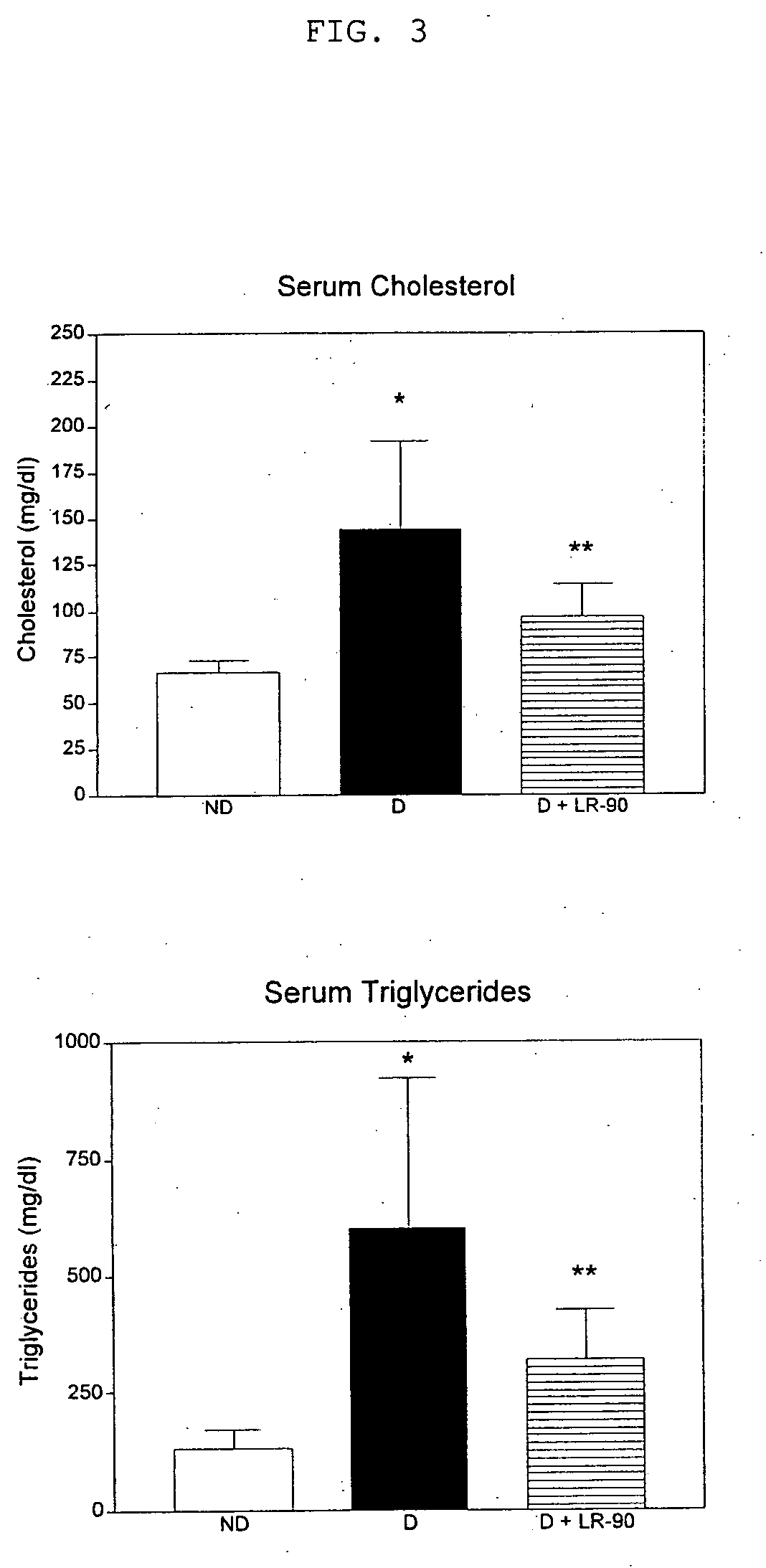
Methods of lowering lipid levels in a mammal
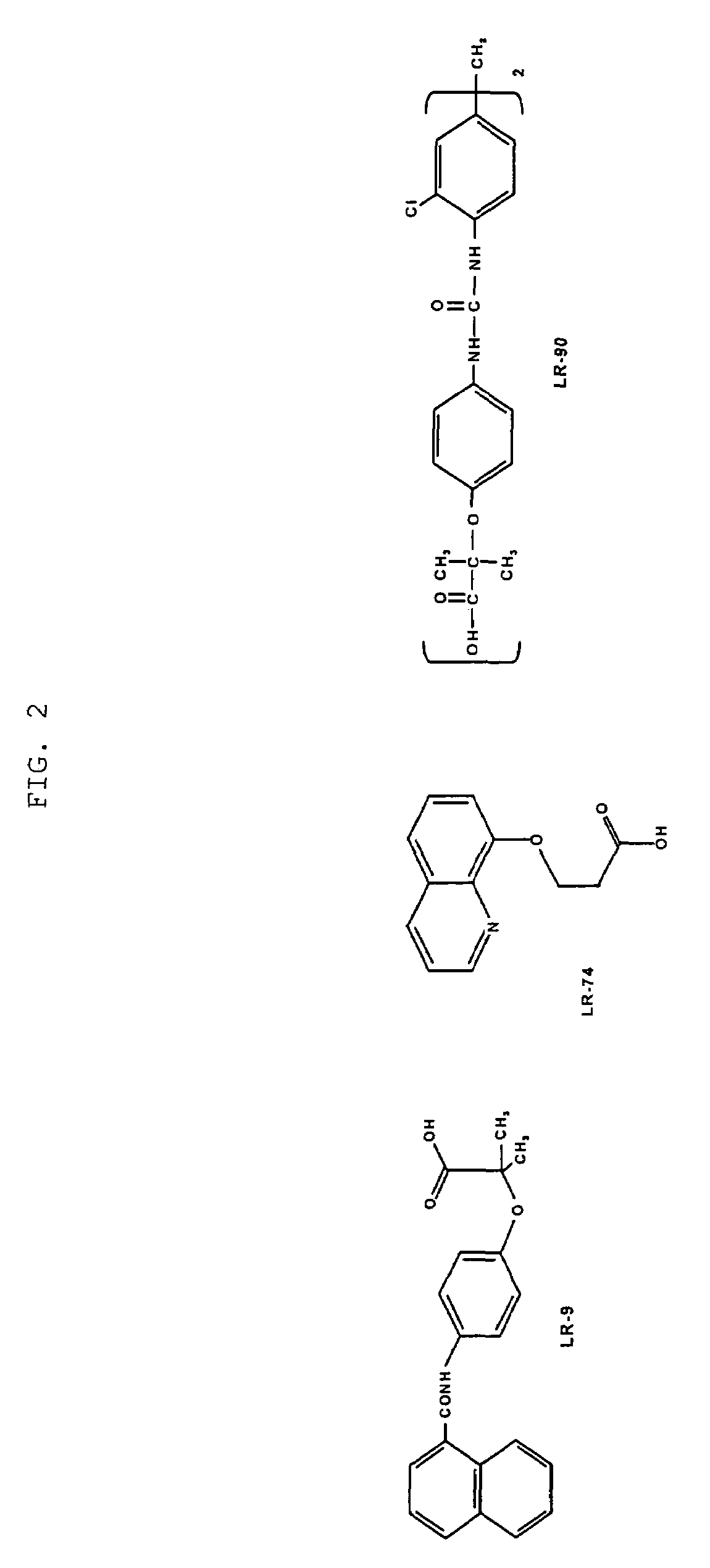
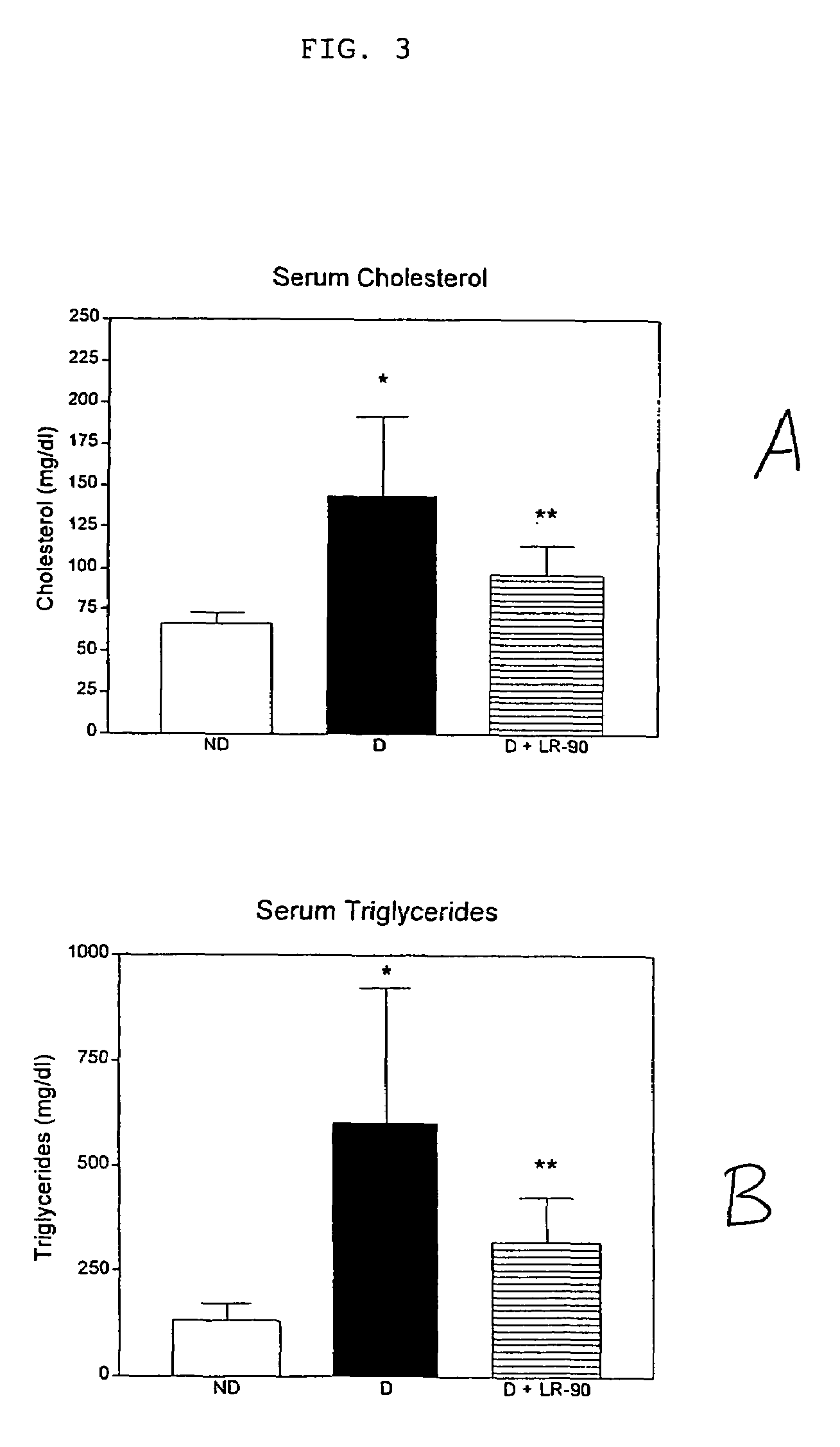
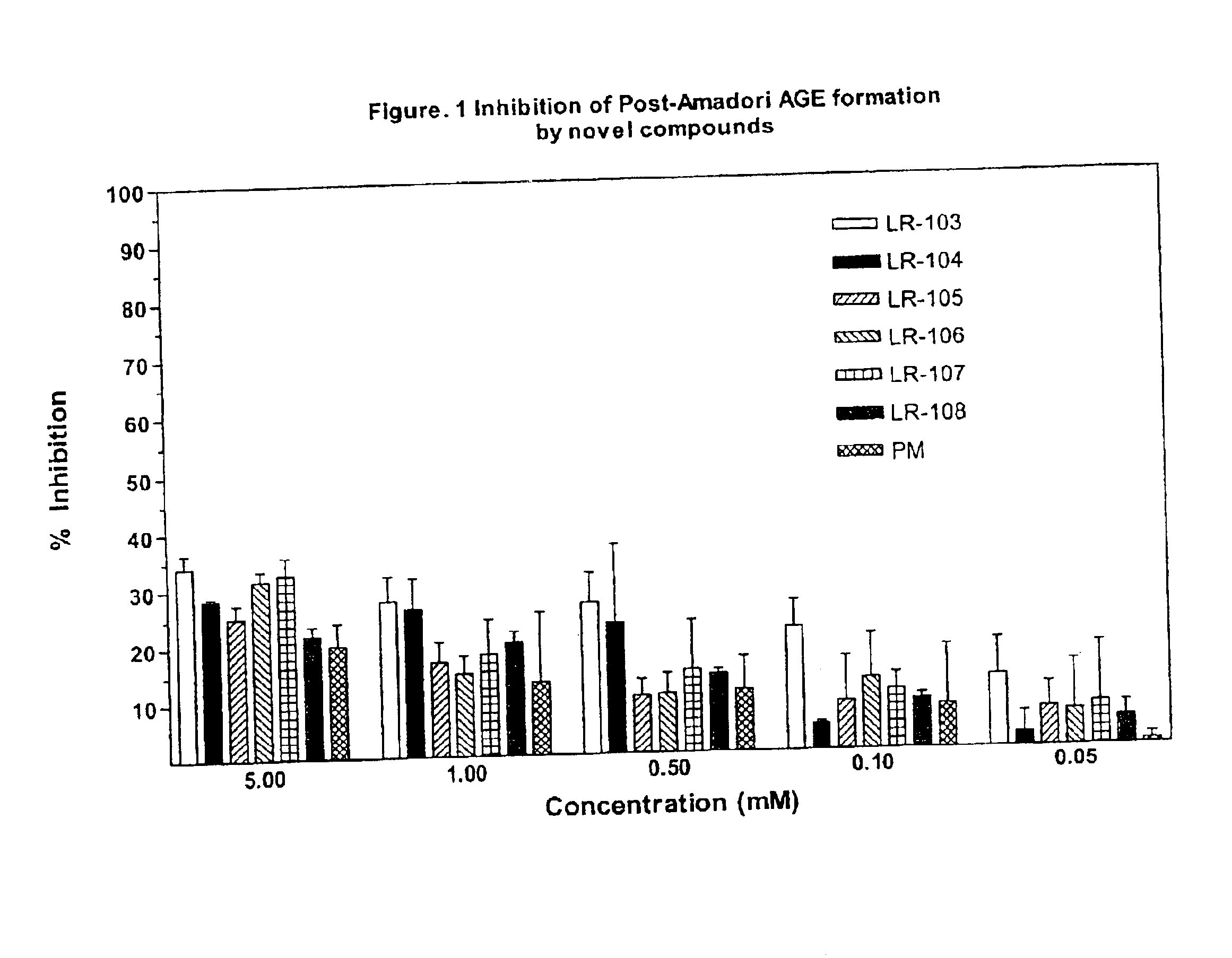
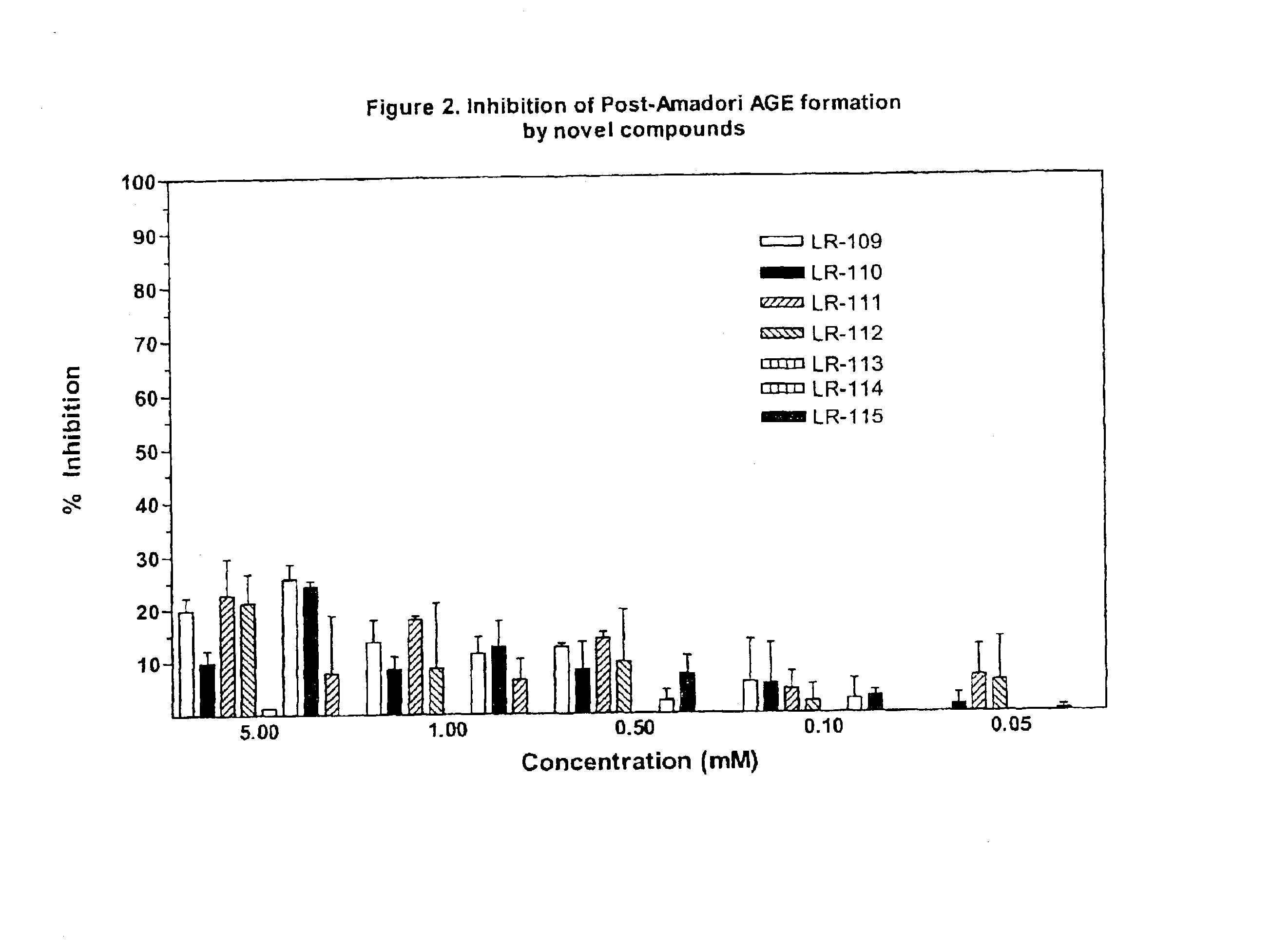
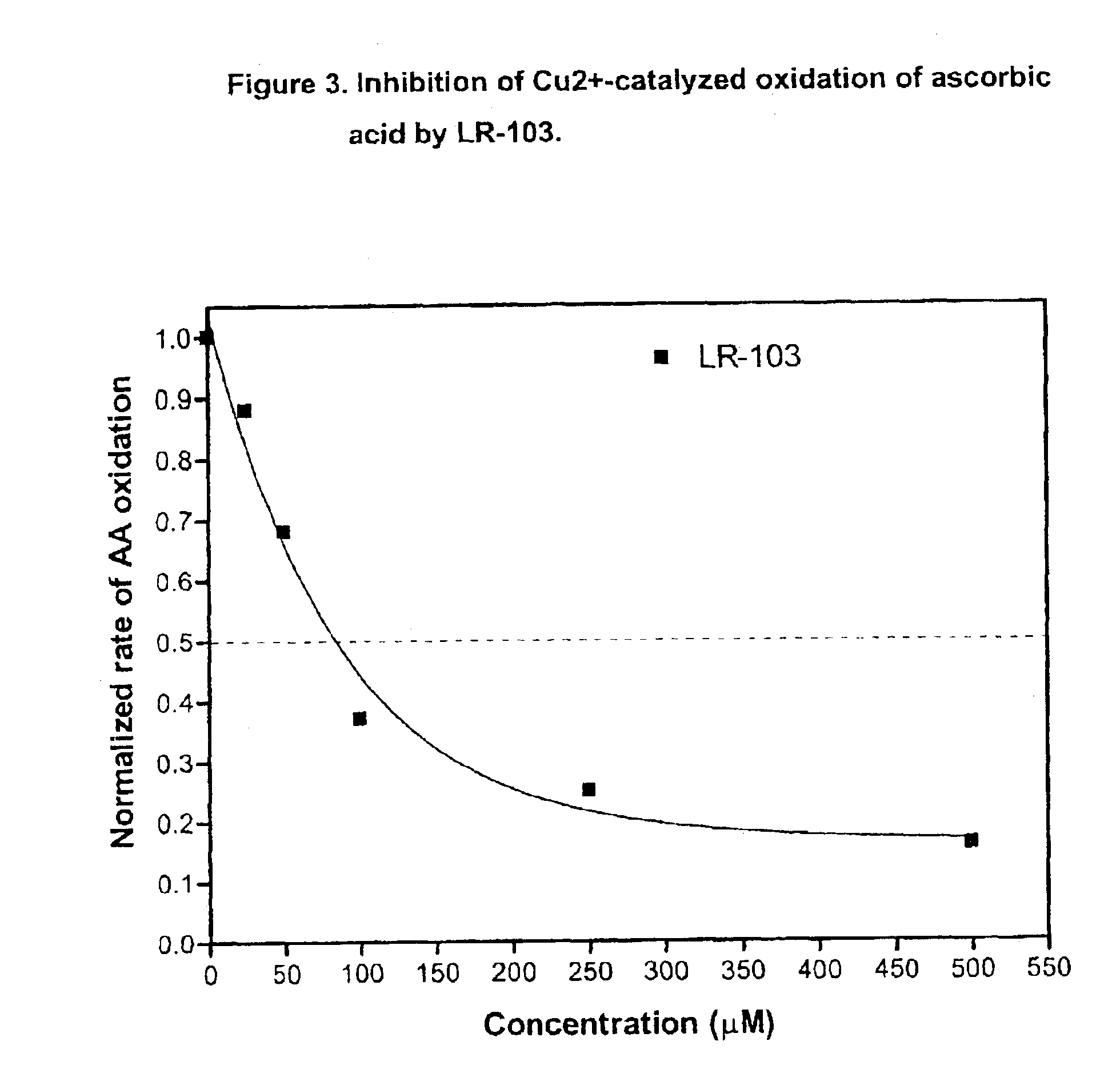
This invention relates to methods for lowering lipid levels in mammals using compounds that inhibit advanced glycation endproducts (AGEs), LR-9, LR-74 and LR-90. These compounds, which inhibit non-enzymatic protein glycation, also inhibit the formation of advanced lipoxidation endproducts (ALEs) on target proteins by trapping intermediates in glycoxidation and lopoxidation and inhibiting oxidation reactions important in the formation of AGEs and ALEs.
Owner:CITY OF HOPE
Methods for Reducing Seizure-Induced Neuronal Damage
InactiveUS20080260717A1Narrow downAlleviate neuronal damageOrganic active ingredientsNervous disorderNeuronal damageMedicine
This invention provides a method for treating a subject either during or soon after a seizure, in order to reduce the extent of neuronal damage in the subject resulting from the seizure comprising administering to the subject, either during or soon after the seizure, a therapeutically effective amount of an inhibitor of receptor for advanced glycation endproducts (RAGE), so as to thereby reduce the extent of neuronal damage in the subject. This invention further provides a method for inhibiting neuronal damage which would otherwise result from a seizure in a subject predisposed to having a seizure, comprising administering to the subject a prophylactically effective amount of an inhibitor of receptor for advanced glycation endproducts (RAGE), so as to inhibit neuronal damage which would otherwise result from a seizure in the event the subject were to suffer a seizure.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
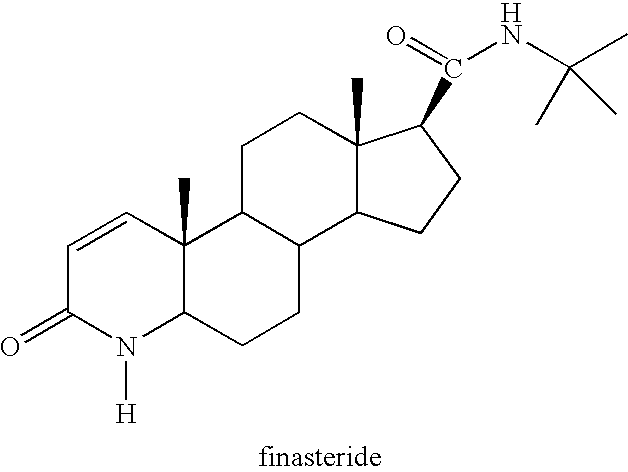
Method of treating men with testosterone supplement and 5alpha-reductase inhibitor
InactiveUS20090123571A1Minimizes unpleasantMinimizes potentially dangerous side effectBiocideOrganic active ingredientsDiseaseAviptadil
A method of treating Alzheimer's disease, Parkinson's disease, sexual dysfunction or erectile dysfunction in a man by administration of a 5alpha reductase inhibitor together with a testosterone supplement is described. The method is also concerned with the use of the 5alpha reductase inhibiting compound and the testosterone supplement together with another agent useful for treating erectile dysfunction, including PDE V inhibitors; AGE (advanced glycation end-product) breakers; alpha 1 blockers; alpha 1A antagonists; alpha 2 antagonists; dopamine agonists; dopamine D4 agonists; melanocortin agonists; oxytocin agonists; prostaglandin; radical scavengers; rotamase inhibitors; aviptadil; nitroglycerine; and GPCR agonists for treating male sexual dysfunction or erectile dysfunction.
Owner:MERCK SHARP & DOHME CORP
Method for treating symptoms of diabetes
InactiveUS7700085B2Inhibit bindingSenses disorderNervous disorderDiabetes mellitusAdvanced Glycation Endproducts
The present invention provides a method for treating symptoms of diabetes in a diabetic subject which comprises administering to the subject a therapeutically effective amount of an agent which inhibits binding of advanced glycation endproducts to any receptor for advanced glycation endproducts so as to treat chronic symptoms of diabetes in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Inhibitors of formation of advanced glycation endproducts (AGEs)
InactiveUS7030133B2Inhibit nonenzymatic glycationIll effectUrea derivatives preparationBiocidePremature agingFunctional integrity
The nonenzymatic glycation and crosslinking of proteins is a part of the aging process with the glycation endproducts and crosslinking of long-lived proteins increasing with age. This process is increased at elevated concentrations of reducing sugars in the blood and in the intracellular environment such as occurs with diabetes. The structural and functional integrity of the affected molecules become disturbed by these modifications and can result in severe consequences. The compounds of the present invention can be used to inhibit this process of nonenzymatic glycation and therefore to inhibit some of the ill effects caused by diabetes or by aging. The compounds are also useful for preventing premature aging, spoilage of proteins in food and can prevent discoloration of teeth.
Owner:CITY OF HOPE
Compounds for the management of aging-related and diabetic vascular complications, process for their preparation, therapeutic and cosmetic uses thereof
InactiveUS7223777B2Compromise membrane integritySensitive to oxidative stressBiocideCosmetic preparationsDiseaseMedicine
The invention discloses a new clause of a five membered heterocylic ring compounds of general formula Iand its pharmaceutically or cosmetically acceptable salts, wherein R1, R2, R3, R4, R5, A, B, X and Y are as defined in the specification. The invention also discloses a process for preparation of these compound and their therapeutic and cosmetic applications particularly in the management of aging related and diabetic vascular complications. The compounds in question act by triple action of an AGE (Advanced Glycation Endproducts) breaker, AGE inhibitor and free radical scavenger which make them most suitable in different therapeutic and cosmetic applications. The invention also discloses pharmaceuticals and cosmetic compositions comprising these compounds and method of treatment of diseases caused by accumulation of AGE and / or free radicals in the body cells.
Owner:TORRENT PHARMA LTD
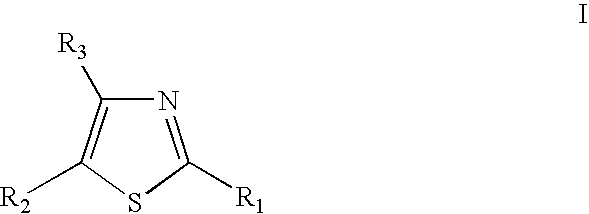
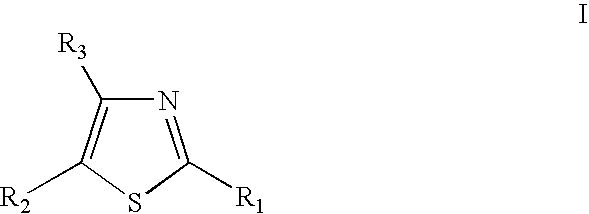
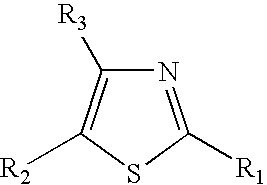
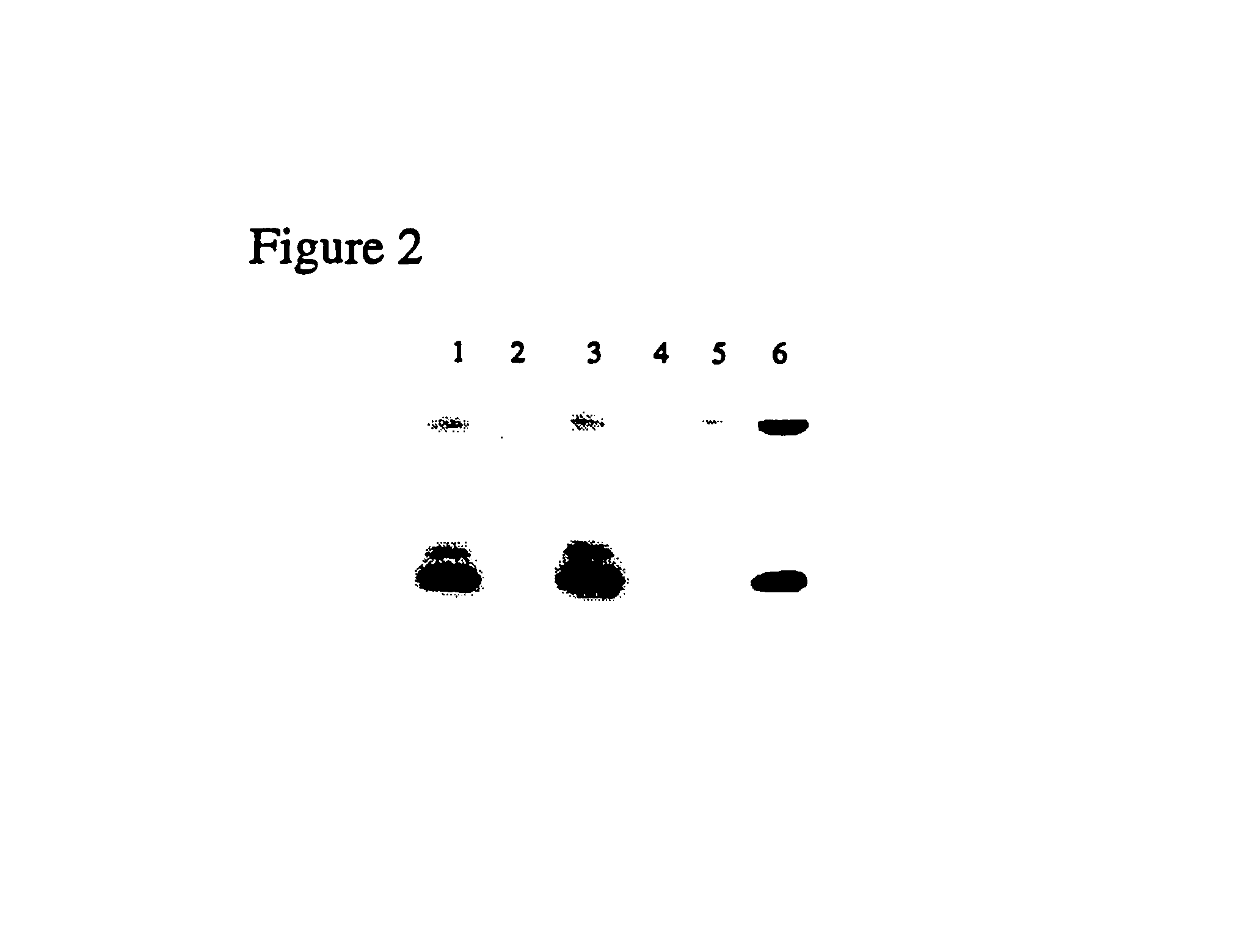
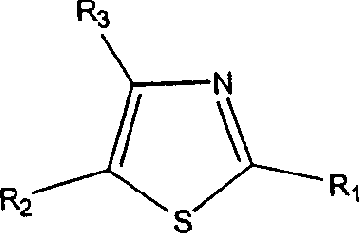
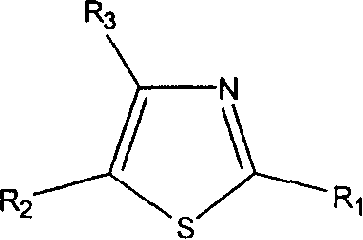
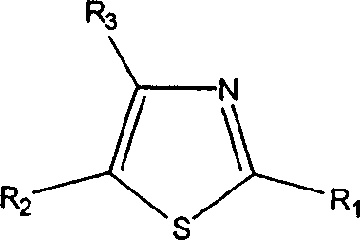
Thiazole derivatives
Methods are disclosed for improving the appearance of skin by topical administration of thiazole derivatives which inhibit the formation of advanced glycation endproducts, break advanced glycation endproduct-associated crosslinks, and inhibit the function of glucose oxidase. Cosmetic and pharmaceutical compositions comprising the thiazole derivatives are also disclosed.
Owner:AVON PROD INC
Methods of lowering lipid levels in a mammal
ActiveUS20050171150A1Reduced increased concentrationAvoid complicationsBiocideNervous disorderLipid formationProtein target
This invention relates to methods for lowering lipid levels in mammals using compounds that inhibit advanced glycation endproducts (AGEs), LR-9, LR-74 and LR-90. These compounds, which inhibit non-enzymatic protein glycation, also inhibit the formation of advanced lipoxidation endproducts (ALEs) on target proteins by trapping intermediates in glycoxidation and lopoxidation and inhibiting oxidation reactions important in the formation of AGEs and ALEs.
Owner:CITY OF HOPE
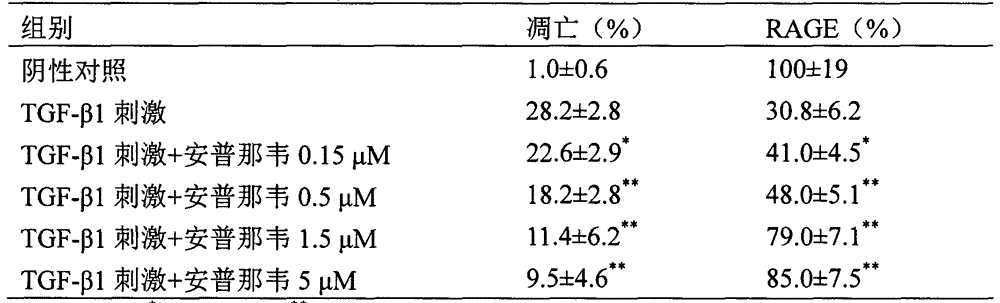
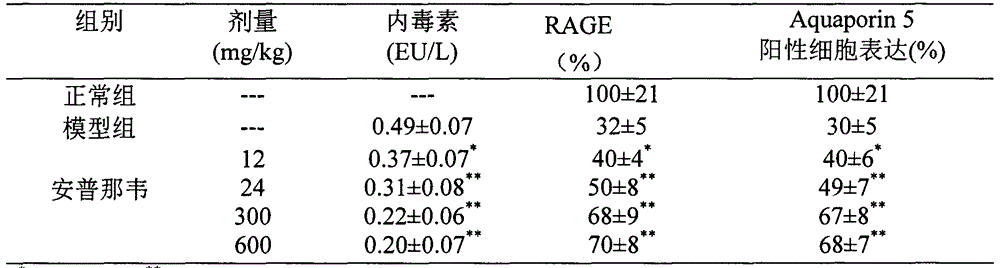
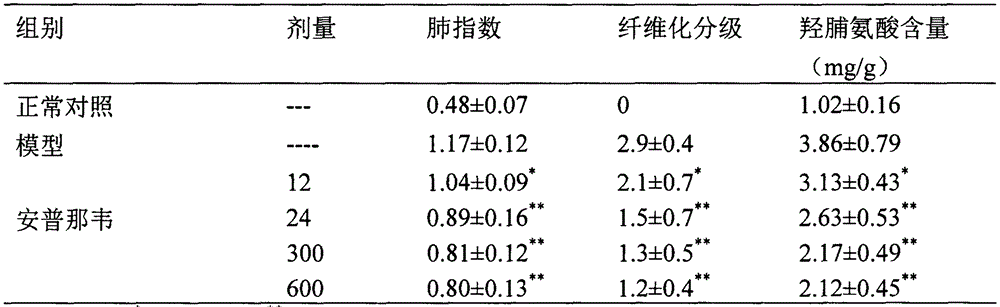
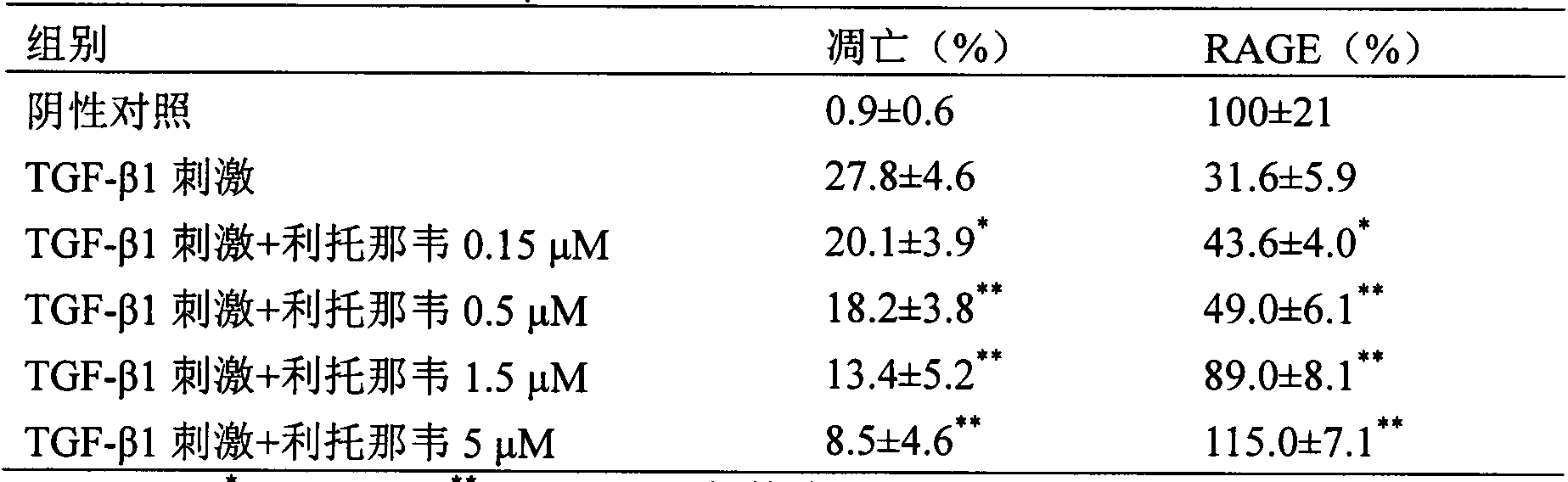
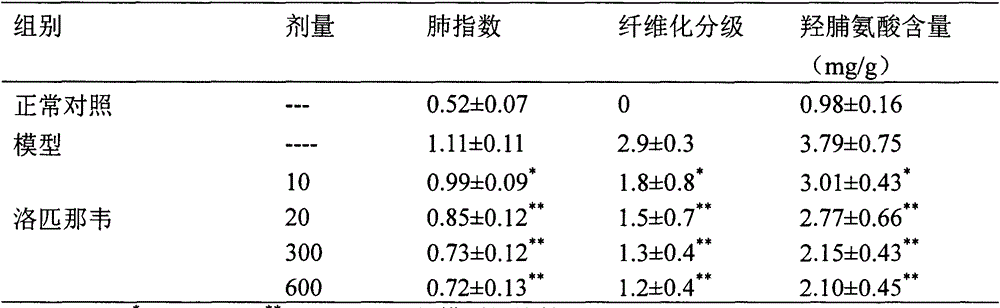
Use of amprenavir in preparation of medicines for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
The invention provides a new medical use of amprenavir and specifically relates to a use of the amprenavir for inhibiting the apoptosis of type I alveolar epithelial cells, promoting the expression of the receptor for advanced glycation endproducts of the type I alveolar epithelial cells, further inhibiting the occurrence and development of the ALI / ARDS and the pulmonary fibrosis and finally preventing or treating acute lung injury / acute respiratory distress syndrome (ALI / ARDS) and pulmonary fibrosis. In the use, the dosage of the amprenavir ranges from 120mg to 6000mg, preferably from 240mg to 3000mg.
Owner:BINZHOU MEDICAL COLLEGE
Extracts of aster koraiensis, and pharmaceutical composition and functional food comprising the same
InactiveCN101678061AInhibitionInhibitory activityMetabolism disorderNatural extract food ingredientsAdditive ingredientN-Butanol
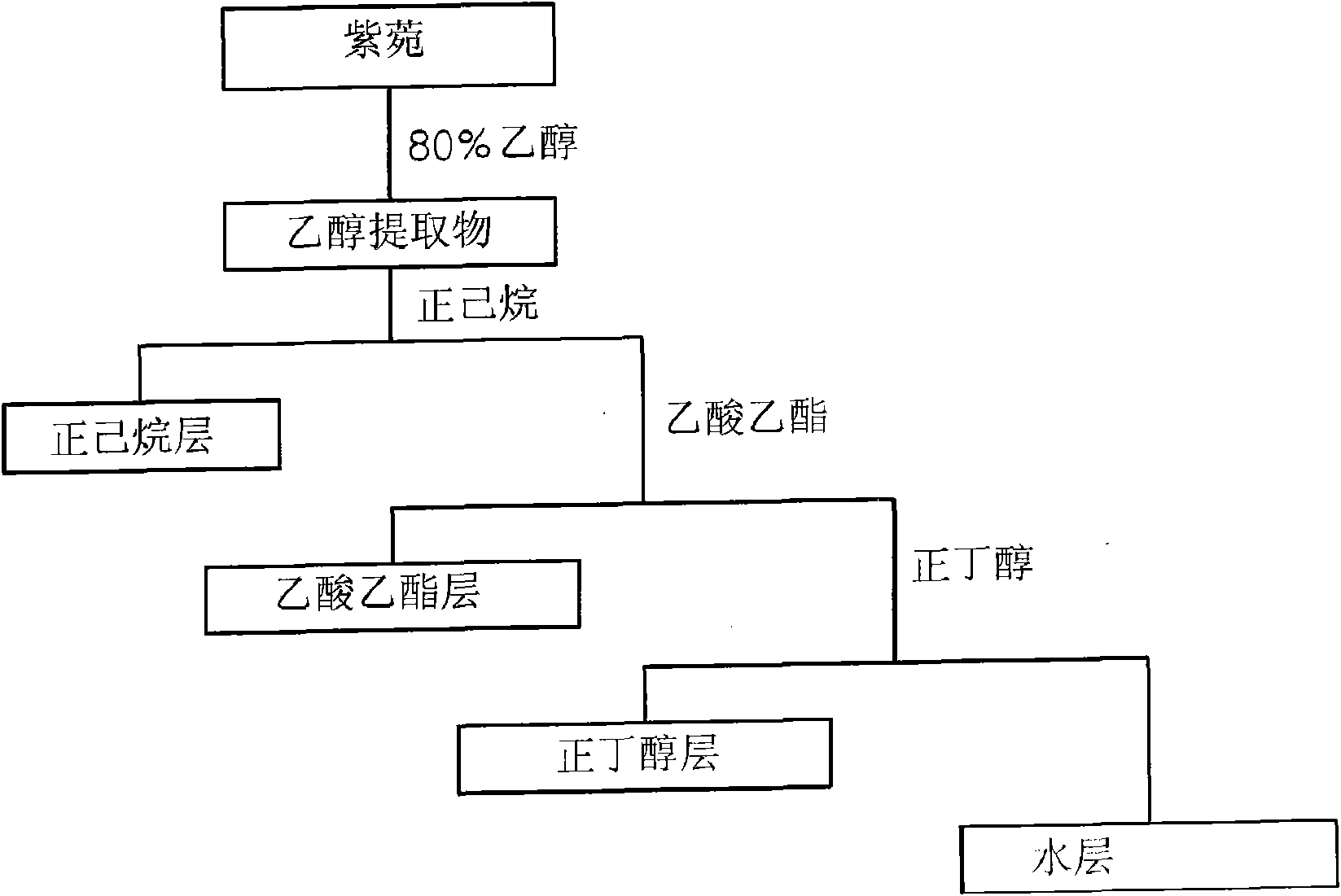
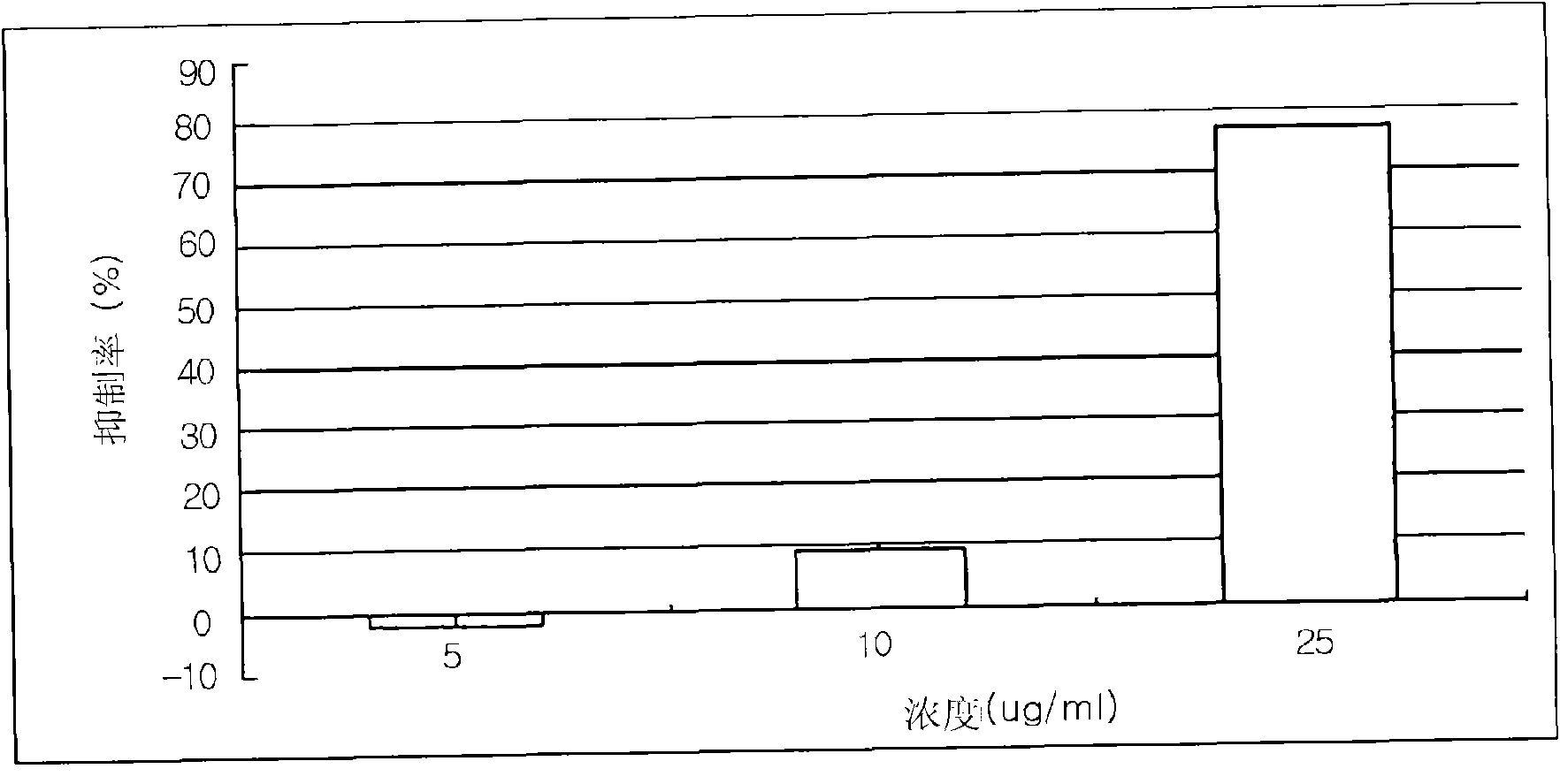
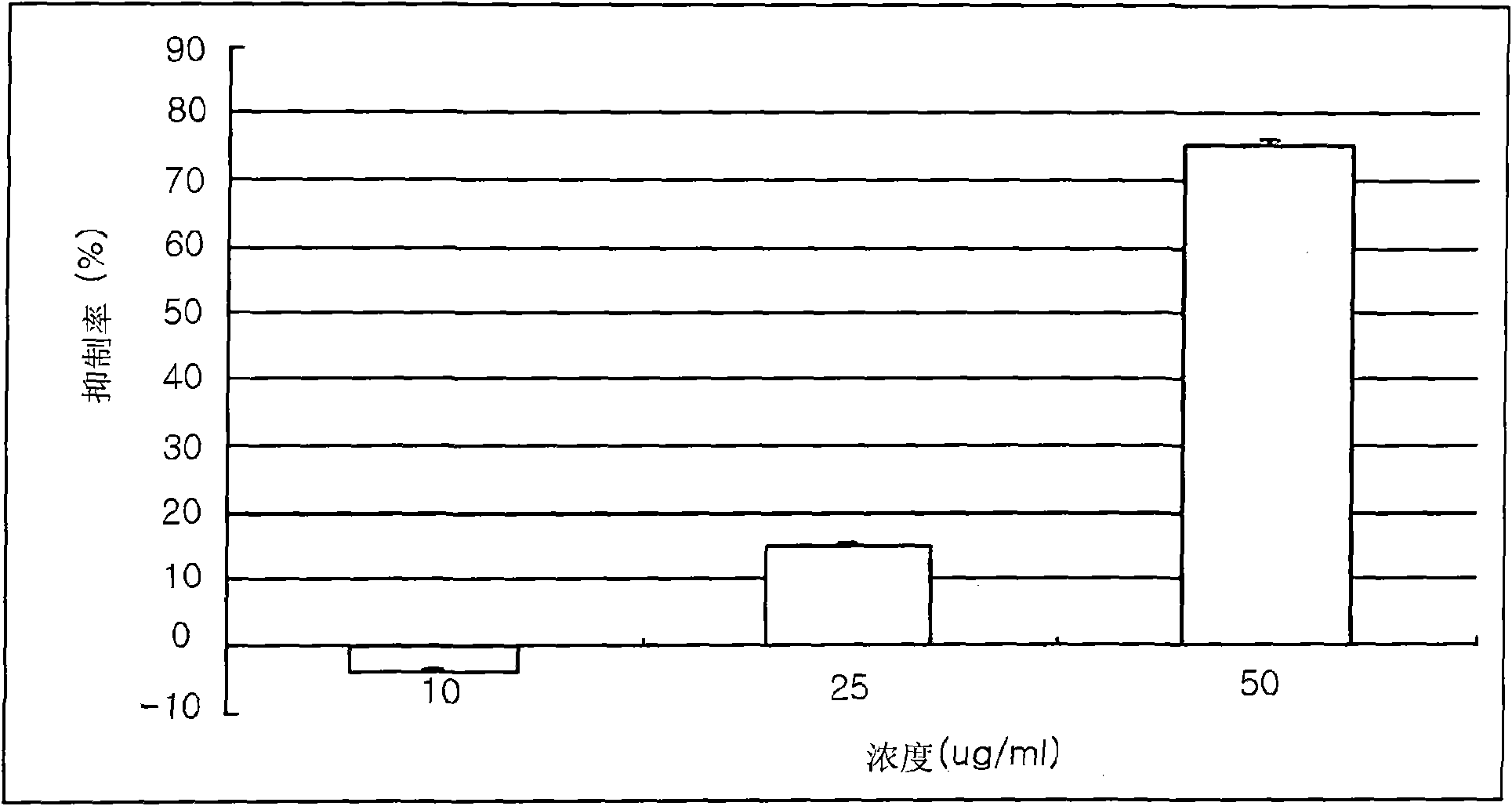
Disclosed herein are a leaf and stem extract of Aster koraiensis, a root extract of Aster koraiensis, a flower extract of Aster koraiensis, a pharmaceutical composition for the prevention or treatment of diabetic complications, a health functional food for the improvement of diabetic complications, and a pharmaceutical composition and health functional food for the prevention or delay of aging, in which each of the compositions and the foods contain, as an active ingredient, at least one of the Aster koraiensis extracts. Each of the extracts is obtained by drying and finely cutting each of the leaf and stem part, root and flower of Aster koraiensis, extracting the cut plant with alcohol or aqueous alcohol solution, filtering the extracted solution and concentrating the filtrate under reduced pressure. Also disclosed are a pharmaceutical composition for the prevention or treatment of diabetic complications, a health functional food for the improvement of diabetic complications, and a pharmaceutical composition and health functional food for the prevention or delay of aging, which contain, as an active ingredient, at least one of fractions obtained by fractionating each of the Aster koraiensis extracts in the order of hexane, ethyl acetate, n-butanol and water fractions. The leaf and stem extract of Aster koraiensis, the root extract of Aster koraiensis and the flower extract of Aster koraiensis have the effect of inhibiting the production of advanced glycation endproducts (AGEs), the causes of diabetic complications, and effectively inhibit aldose reductase (AR) activity. Thus, the disclosed extracts will be highly useful for the prevention or treatment of diabetic complications and the prevention of aging.
Owner:KOREA INST OF ORIENTAL MEDICINE
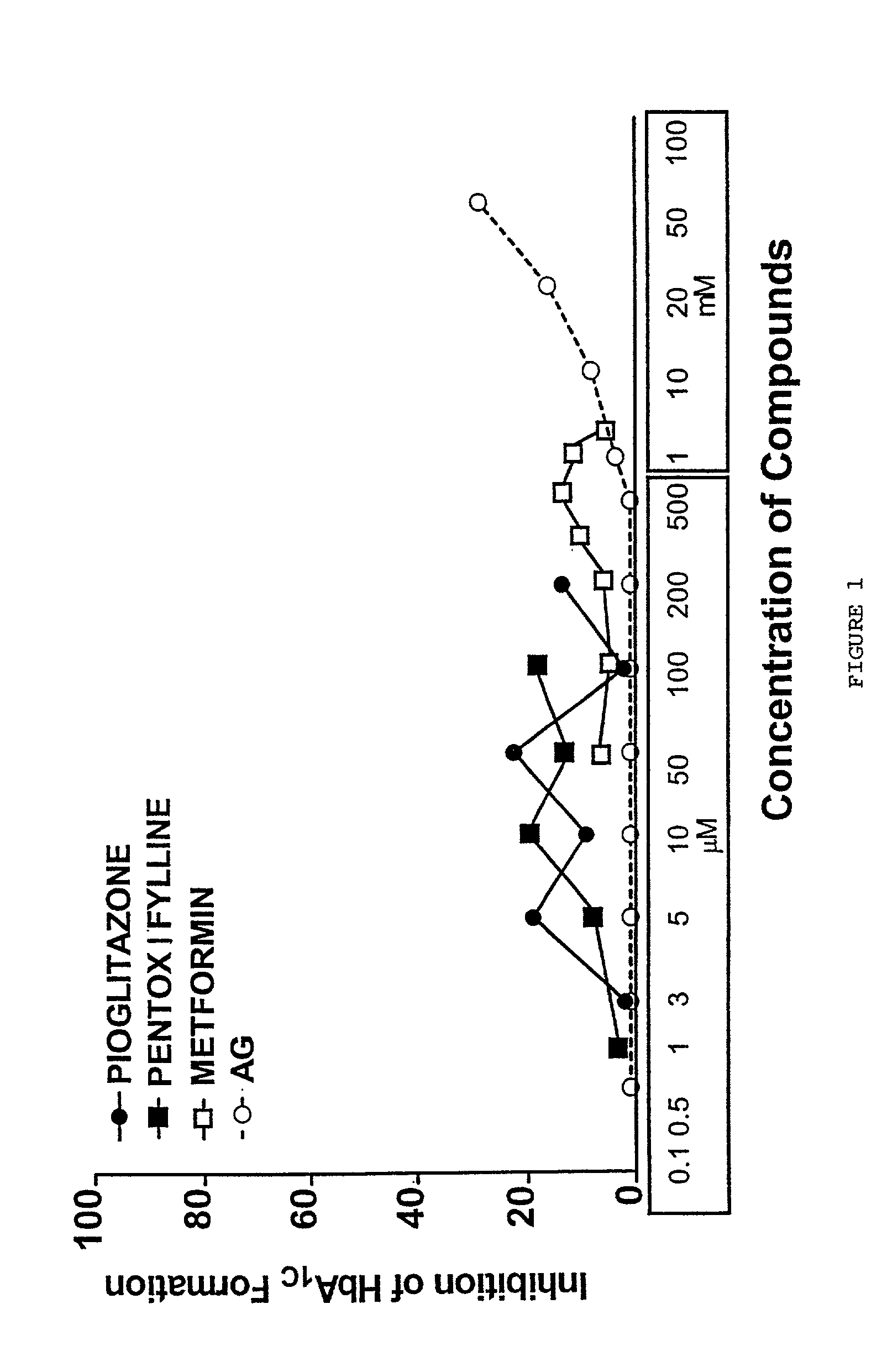
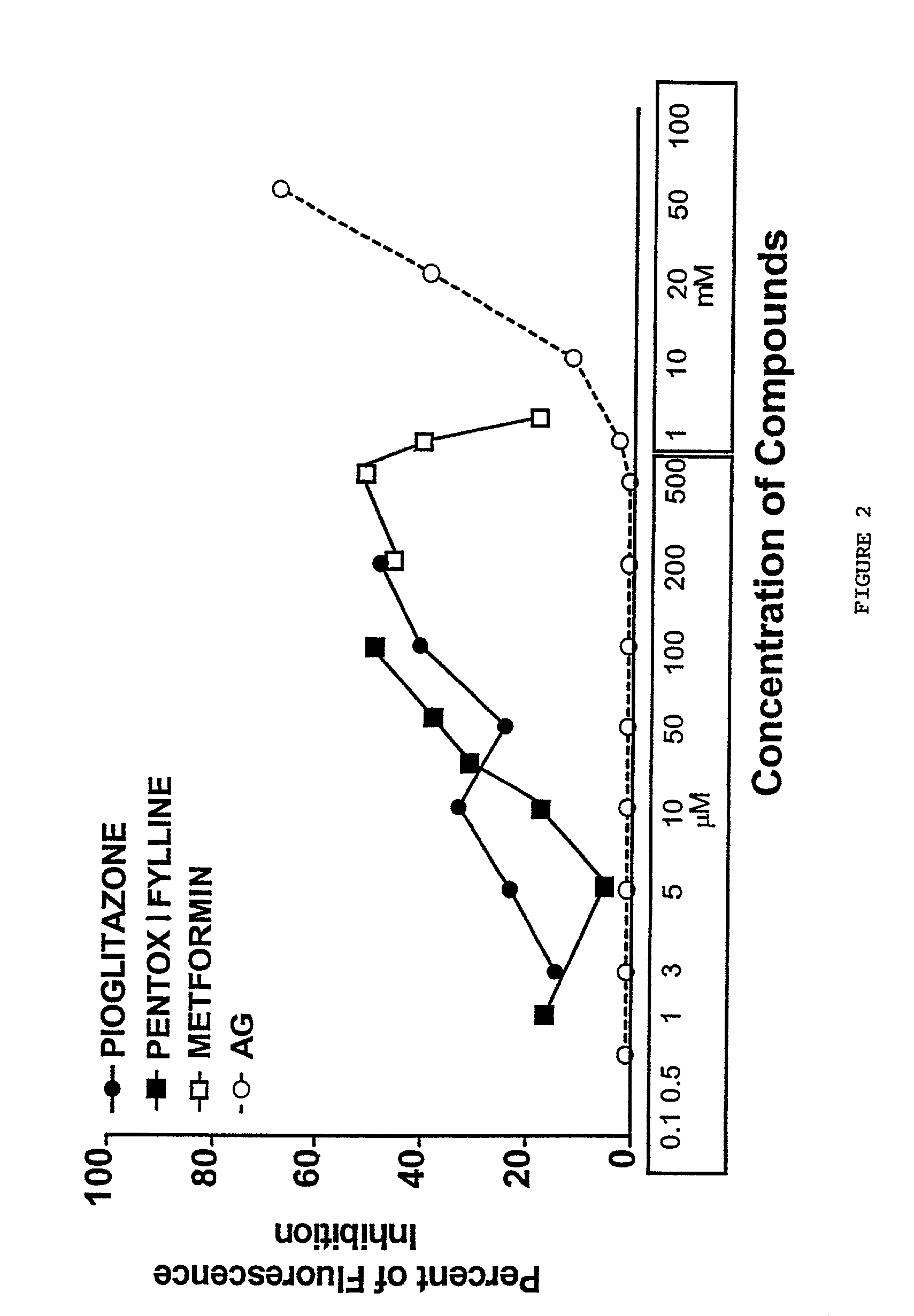
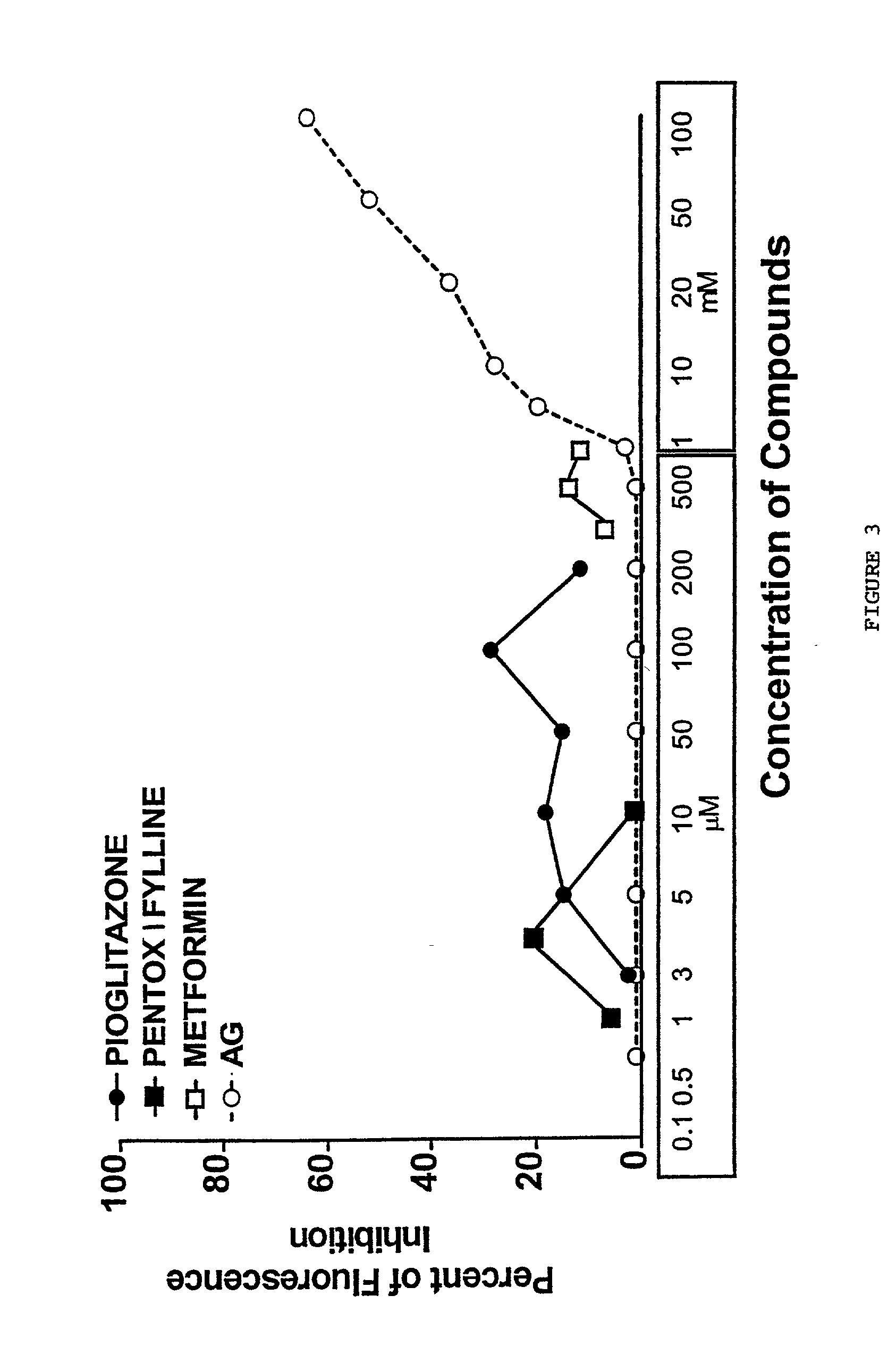
Pentoxifylline, pioglitazone and metformin are inhibitors of formation of advanced glycation endproducts (AGE's)
InactiveUS20020128278A1Inhibit nonenzymatic glycationIll effectBiocideNervous disorderDiseasePremature aging
Pentoxifylline, pioglitazone and metformin have been found to inhibit the nonenzymatic glycation of proteins which often results in formation of advanced glycation endproducts and crosslinks. The nonenzymatic glycation and crosslinking of proteins is a part of the aging process with the glycation endproducts and crosslinking of long-lived proteins increasing with age. This process is increased at elevated concentrations of reducing sugars in the blood and in the intracellular environment such as occurs with diabetes. The structural and functional integrity of the affected molecules become perturbed by these modifications and can result in severe consequences. The compounds of the present invention can be used to inhibit this process of nonenzymatic glycation and therefore to inhibit some of the ill effects caused by diabetes or by aging. The compounds are also useful for preventing premature aging, rheumatoid arthritis, Alzheimer's disease, uremia, neurotoxicity, atherosclerosis and spoilage of proteins in food and can prevent discoloration of teeth.
Owner:CITY OF HOPE
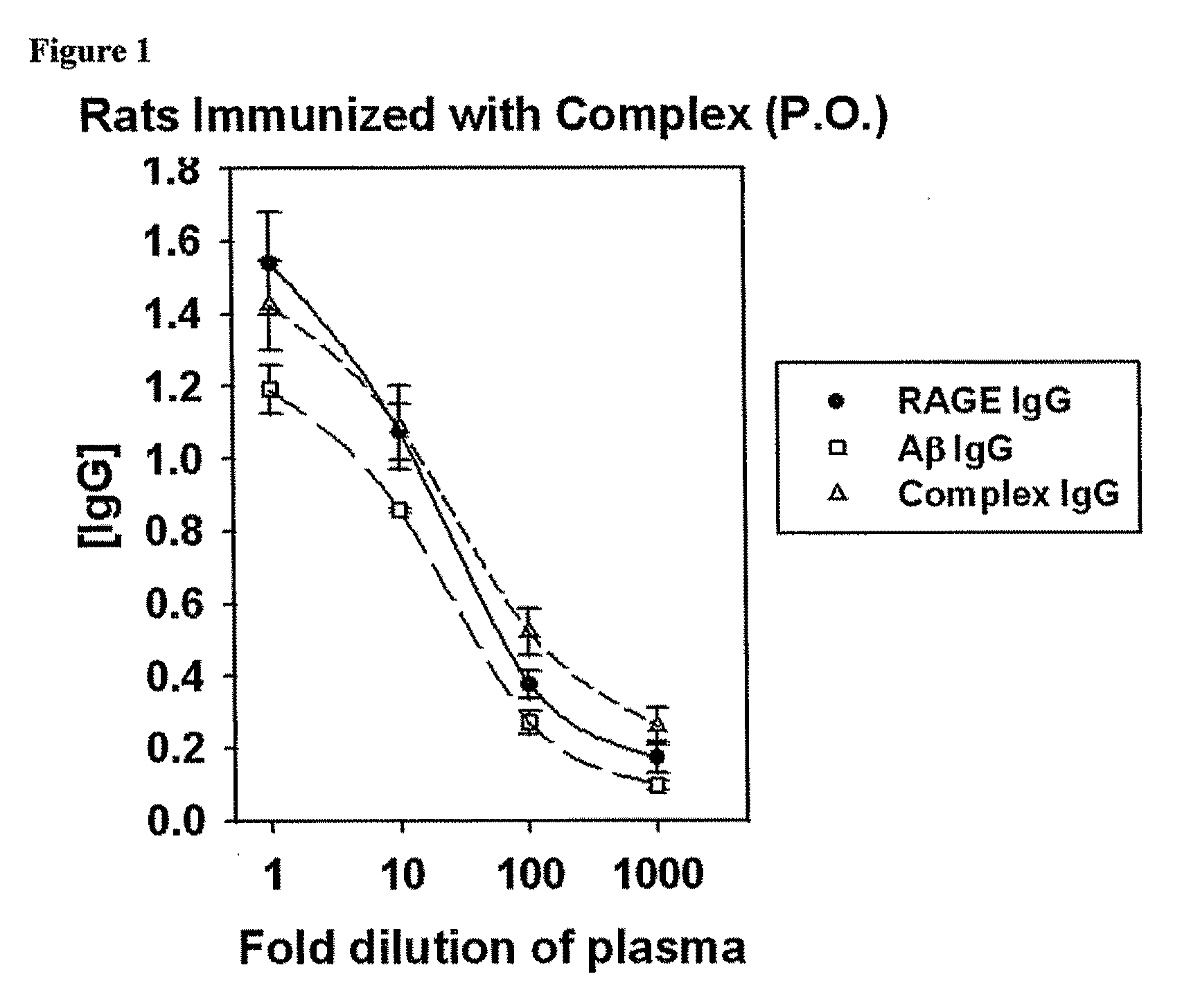
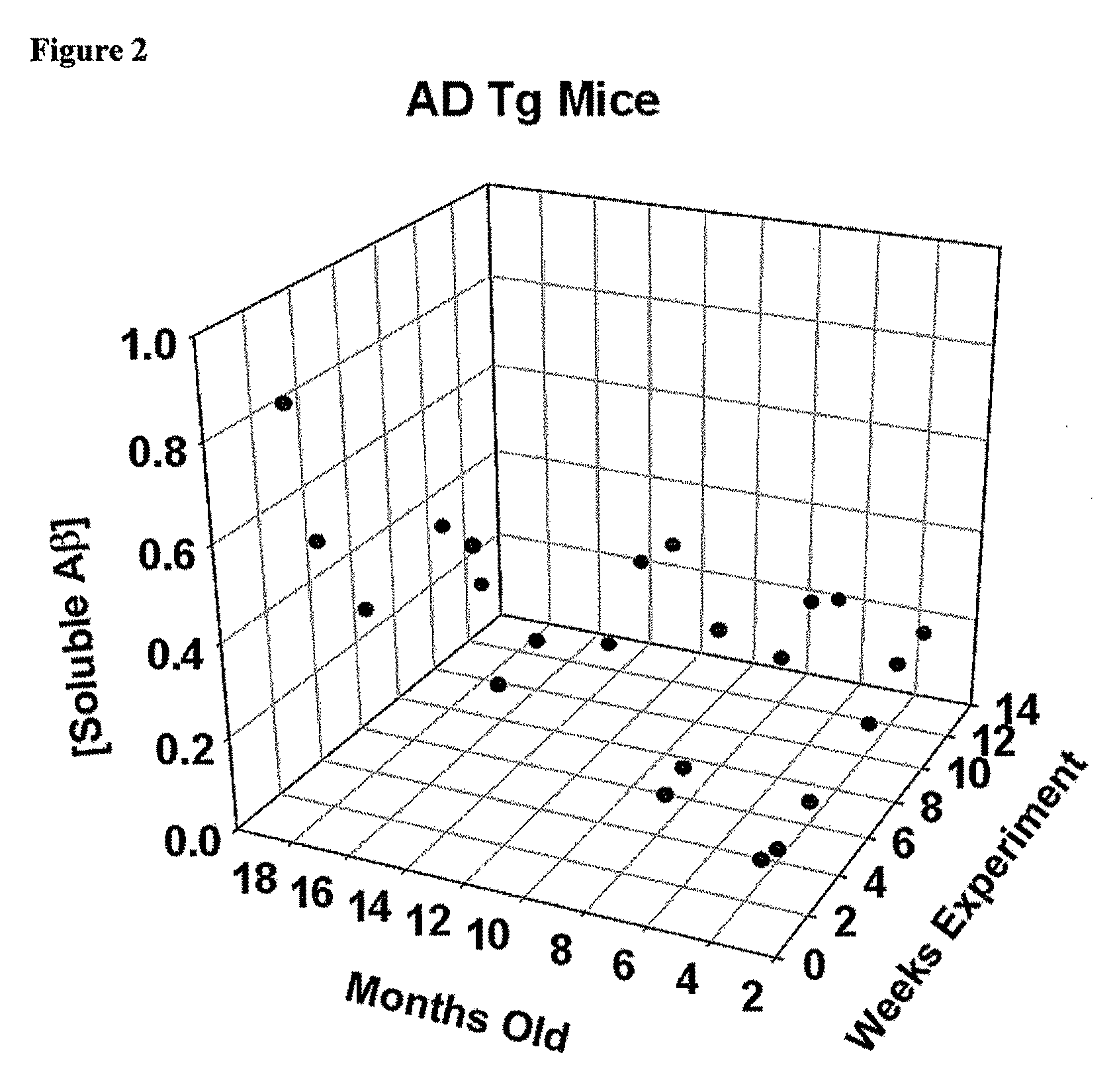
High Molecular Weight Amyloid Beta As a Carrier for the Oral Delivery of Vaccine Antigens
InactiveUS20100297160A1Provoke immune responseNervous disorderSnake antigen ingredientsImmunogenicityAdvanced Glycation Endproducts
Compositions and methods are provided for stabilizing polypeptide antigens such as amyloid-beta (Aβ) to produce vaccines for oral delivery. One embodiment provides an immunogenic polypeptide complex of Aβ42 and an fragment of receptor for advanced glycation endproducts (RAGE).
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
Control effect and mechanism of mangiferin on diabetic encephalopathy
InactiveCN102813645ANew pharmacological effectNew medical useOrganic active ingredientsNervous disorderWhite blood cellPharmacologic action
The invention relates to novel pharmacologic action of mangiferin, i.e., effects of mangiferin on preventing and treating diabetic encephalopathy. Mangiferin can obviously improve cognitive impairment caused by diabetes, remarkably reduce the level of advanced glycation endproducts (AGF) in the brain and expression of the acceptor RAGE thereof, substantially enhance activity and expression of glyoxalase 1 (Glo-1) in the brain, remarkably reduce the level of interleukin 1-beta (IL-1beta) and tumor necrosis factor alpha (TNF-alpha) in the brain, substantially improve the level of reduced glutathione (GSH) in the brain, remarkably reduce the level of malonaldehyde (MDA) in the brain, substantially improve the activity of superoxide dismutase (SOD) and the level of GSH in serum and substantially reduce the level of MDA in the serum. Mangiferin can be used for preventing and treating diabetic complications like diabetic encephalopathy.
Owner:XUZHOU MEDICAL COLLEGE
Method of treating men with testosterone supplement and 5alpha-reductase inhibitor
A method of treating Alzheimer's disease, Parkinson's disease, sexual dysfunction or erectile dysfunction in a man by administration of a 5alpha reductase inhibitor together with a testosterone supplement is described. The method is also concerned with the use of the 5alpha reductase inhibiting compound and the testosterone supplement together with another agent useful for treating erectile dysfunction, including PDE V inhibitors; AGE (advanced glycation end-product) breakers; alpha 1 blockers; alpha 1A antagonists; alpha 2 antagonists; dopamine agonists; dopamine D4 agonists; melanocortin agonists; oxytocin agonists; prostaglandin; radical scavengers; rotamase inhibitors; aviptadil; nitroglycerine; and GPCR agonists for treating male sexual dysfunction or erectile dysfunction.
Owner:MERCK & CO INC
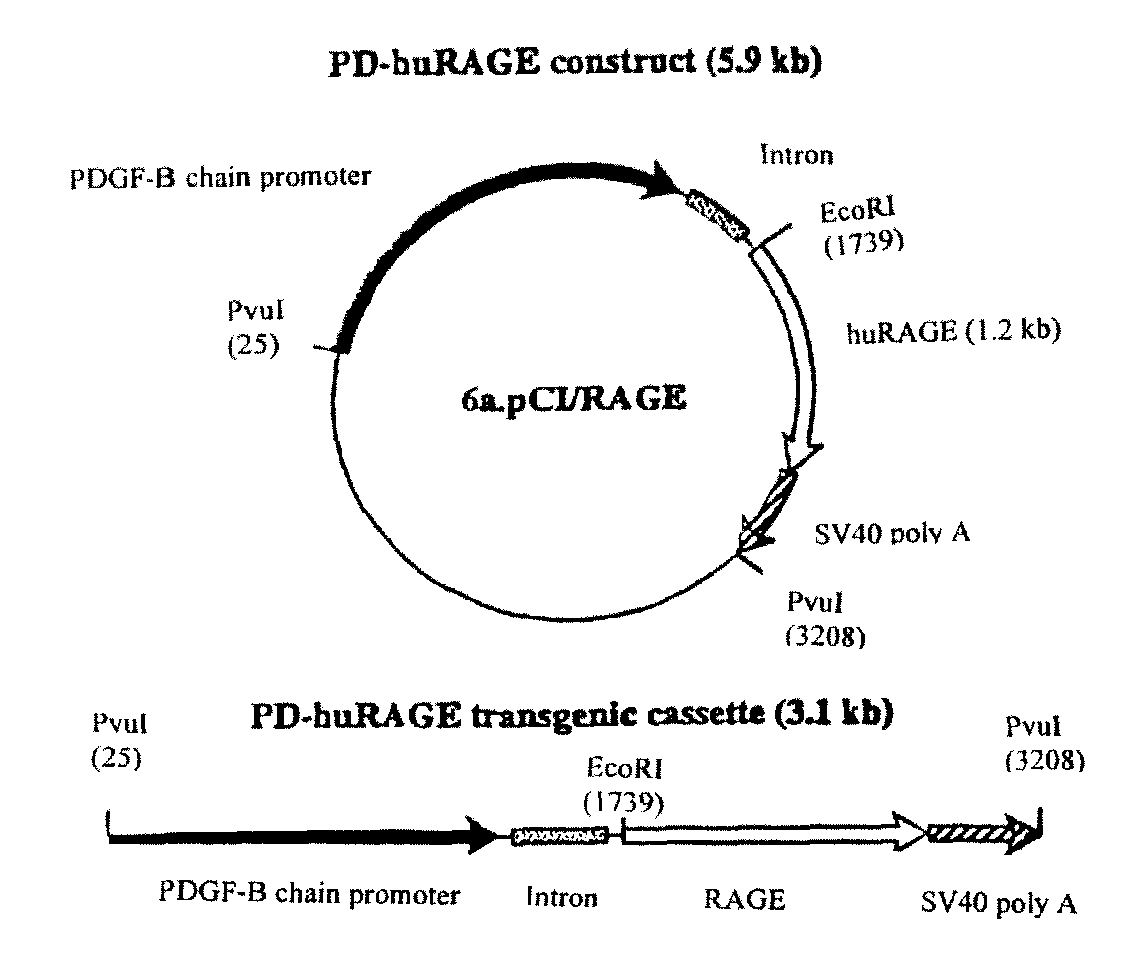
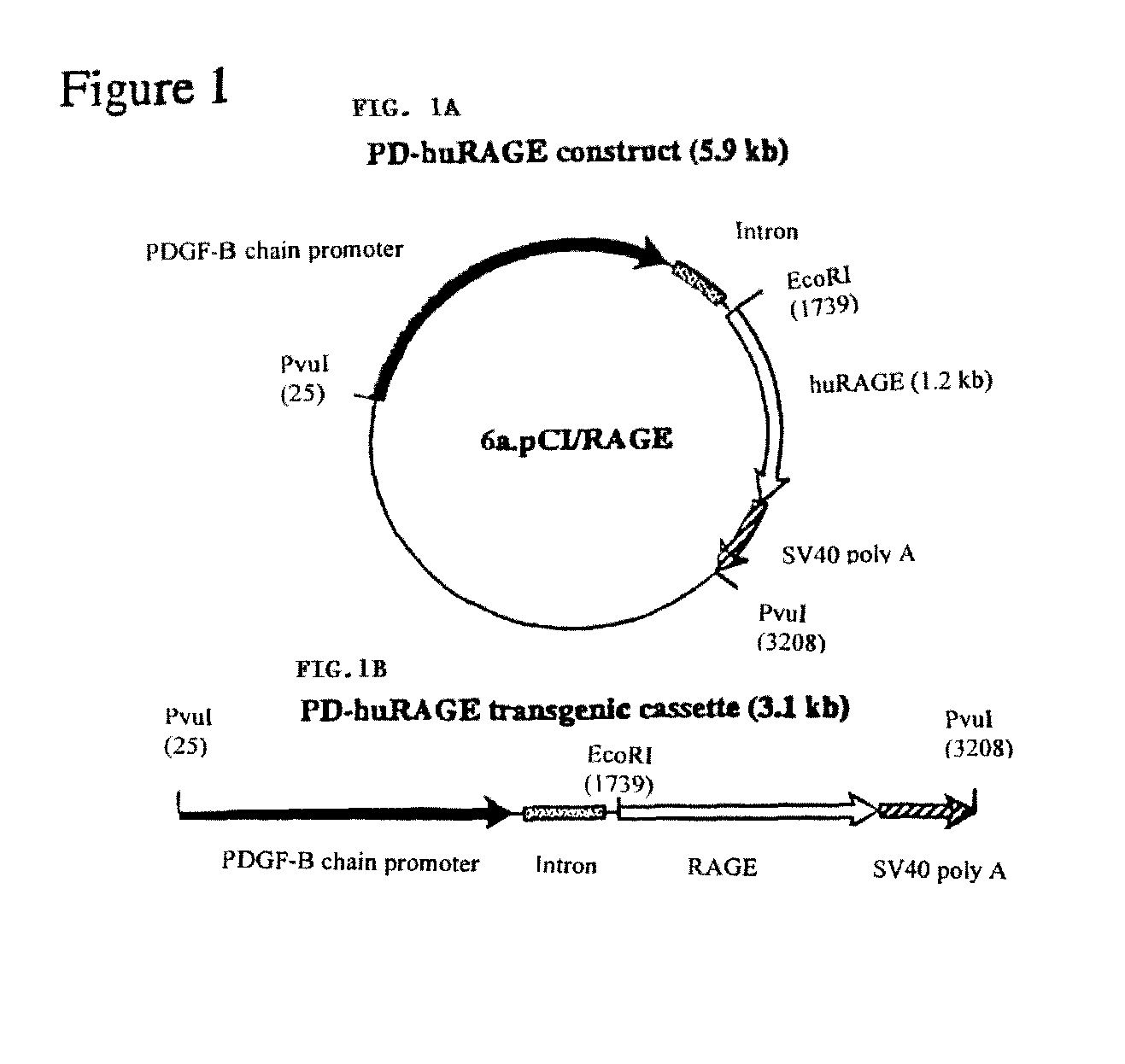
Transgenic mice over-expressing receptor for advanced glycation endproduct (RAGE) in brain and uses thereof
The present invention provides for a transgenic non-human animal whose cells contain a DNA sequence comprising: (a) a nerve tissue specific promoter; and (b) a DNA sequence which encodes a receptor for advanced glycation endproducts (RAGE), wherein the promoter and the DNA sequence which encodes the receptor for advanced glycation endproducts (RAGE) are operatively linked to each other and integrated in the genome of the non-human animal, and wherein said non-human animal exhibits a reduced amount of cerebral tissue infarcted following a transient middle cerebral artery occlusion compared to an identical non-human animal lacking said DNA sequence.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Thiazole derivatives
InactiveCN1636563AOrganic active ingredientsCosmetic preparationsDermatomalPharmaceutical Substances
Disclosed are methods of improving the appearance of skin by topical administration of thiazole derivatives that inhibit the formation of advanced glycation end products, cleave crosslinks associated with advanced glycation end products, and inhibit the function of glucose oxidase . Cosmetic and pharmaceutical compositions comprising said thiazole derivatives are also disclosed.
Owner:AVON PROD INC
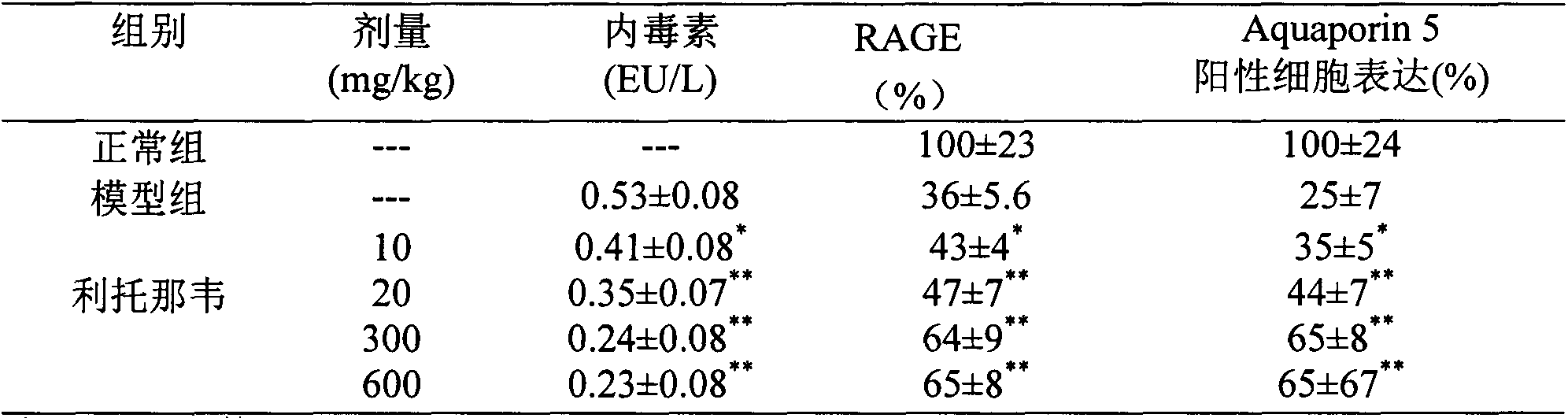
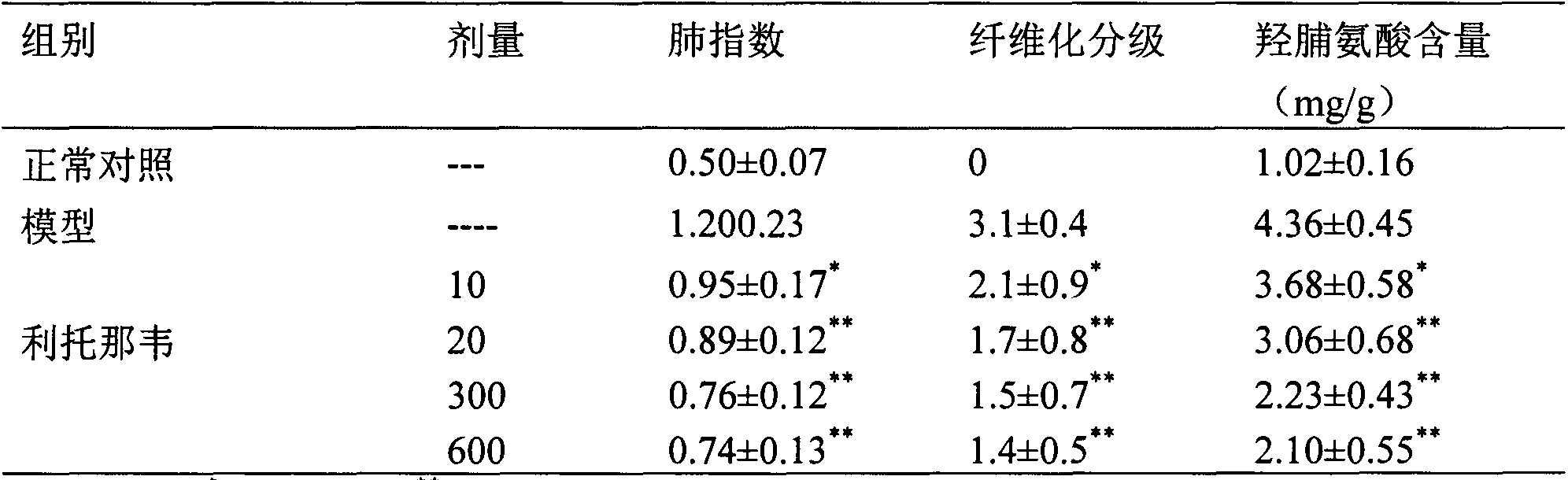
Application of ritonavir in preparing medicines for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
InactiveCN104069104AOrganic active ingredientsRespiratory disorderApoptosisARDs - Acute respiratory distress syndrome
The invention provides a new medical application of ritonavir and in particular relates to an application of the ritonavir for preventing or treating Acute Lung Injury / Acute Respiratory Distress Syndrome (ALI / ARDS) and pulmonary fibrosis in such a manner of inhibiting the occurrence and development of the ALI / ARDS and the pulmonary fibrosis by inhibiting the apoptosis of type I alveolar epithelial cells and promoting the expression of the Receptor For Advanced Glycation Endproducts (RAGE) of the type I alveolar epithelial cells. When in use, the oral administration dosage of the ritonavir ranges from 100mg to 6000mg, preferably, from 200mg to 3000mg.
Owner:BINZHOU MEDICAL COLLEGE
Piperidine derivatives useful for treatment of diebetes
Owner:TORRENT PHARMA LTD
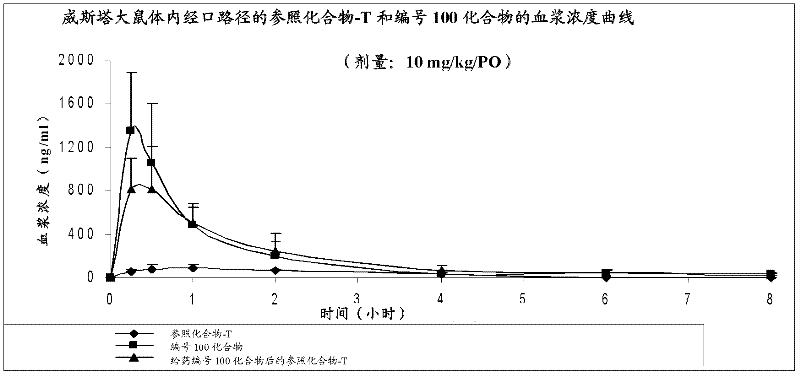
Use of lopinavir in preparation of medicines for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
InactiveCN104083373AOrganic active ingredientsRespiratory disorderApoptosisARDs - Acute respiratory distress syndrome
The invention provides a new medical use of lopinavir and specifically relates to a use of the lopinavir in preventing or treating acute lung injury / acute respiratory distress syndrome (ALI / ARDS) and pulmonary fibrosis by inhibiting the apoptosis of type I alveolar epithelial cells, promoting the expression of the receptor for advanced glycation endproducts of the type I alveolar epithelial cells, further inhibiting the occurrence and development of ALI / ARDS and the pulmonary fibrosis and finally preventing or treating ALI / ARDS. In the use, the dosage of the lopinavir ranges from 100mg to 6000mg, preferably from 240mg to 3000mg.
Owner:BINZHOU MEDICAL COLLEGE
Method for treating symptoms of diabetes
InactiveUS20100255081A1BiocideSenses disorderAdvanced Glycosylation End ProductsAdvanced Glycation Endproducts
The present invention provides a method for treating symptoms of diabetes in a diabetic subject which comprises administering to the subject a therapeutically effective amount of an agent which inhibits binding of advanced glycation endproducts to any receptor for advanced glycation endproducts so as to treat chronic symptoms of diabetes in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com