Application of ritonavir in preparing medicines for preventing or treating acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis
A technology for ritonavir and pulmonary fibrosis, applied in the field of application of ritonavir in the preparation of drugs for the prevention or treatment of acute lung injury/acute respiratory distress syndrome and pulmonary fibrosis, can solve the pharmacological effects that have not been seen Reporting, slowing the spread of HIV, and more
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0012] Embodiment 2 Ritonavir tablet preparation
[0013] Weigh 100.0g ritonavir and 100.0g carboxymethyl starch sodium, mix well and pass through a 100 mesh sieve, add an appropriate amount of 3% PVP k30 Appropriate amount of water solution to make soft material, granulate with 20-mesh sieve, dry at 60°C for 3 hours, granulate with 18-mesh sieve, add 2.0g of magnesium stearate, mix well and press into tablets, adjust the weight of the tablet to about 200mg.
experiment example 2
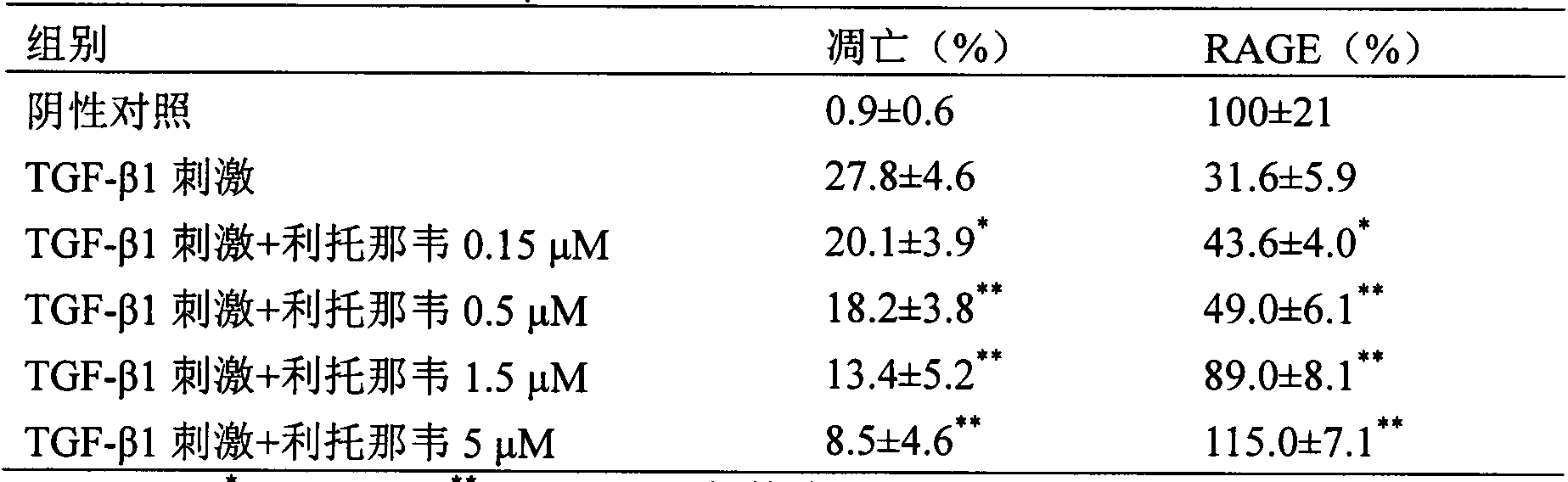
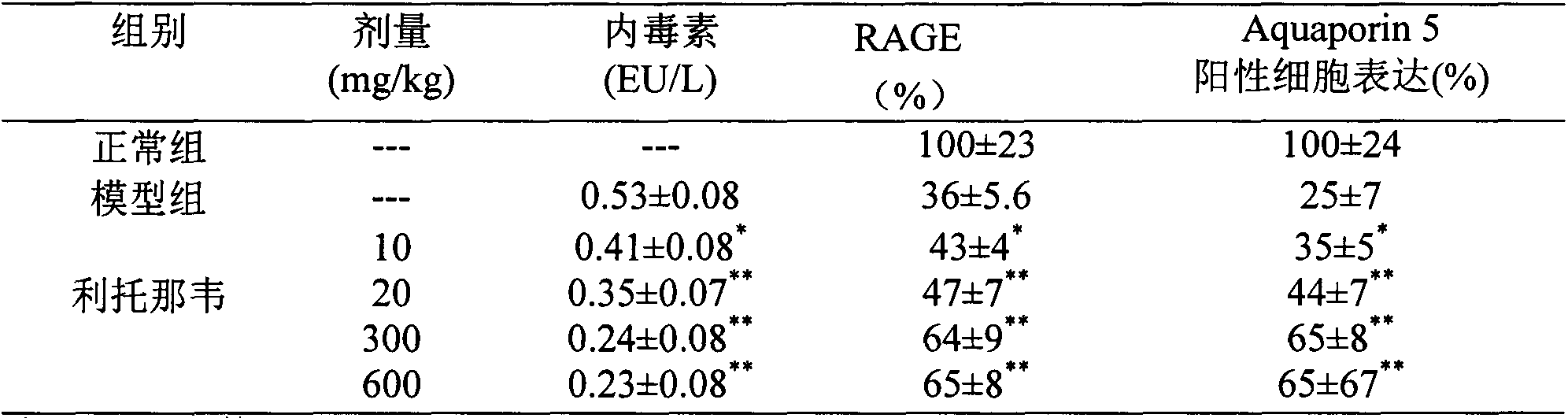
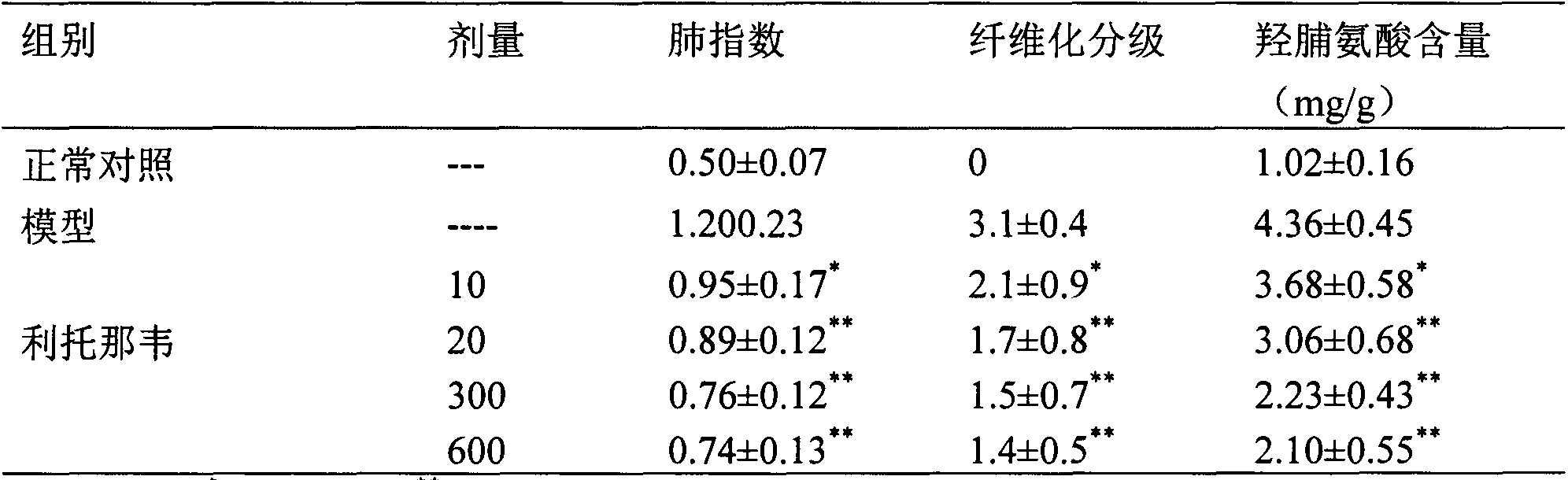
[0025]Experimental example 2 Effect of ritonavir on acute lung injury / acute respiratory distress syndrome (ALI / ARDS) model rats caused by cecal ligation and perforation
[0026] 2.1 Drugs and reagents
[0027] Ritonavir (purity 99.5%, purchased from Dalian Meilun Biotechnology Co., Ltd.)
[0028] Limulus reagent kit (Fuzhou Xinbei Biochemical Industry Co., Ltd., Fujian Province, batch number: 080430)
[0029] Anti-Aquaporin5 antibody (abcam company, ab104751)
[0030] RAGE primary antibody was purchased from Sigma.
[0031] Experimental animals: SPF grade Sprague Dawley rats, male, weighing 150g-200g, provided by the Experimental Animal Center of Shandong Luye Pharmaceutical Co., Ltd., the animal qualification certificate number is: SYXK (Lu) 20030020.
[0032] 2.2 Experimental methods and results
[0033] 2.2.1 Preparation of rats with cecal ligation and puncture (CLP)
[0034] On the day of the operation, the rats were fasted in the morning, placed in the supine positio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com