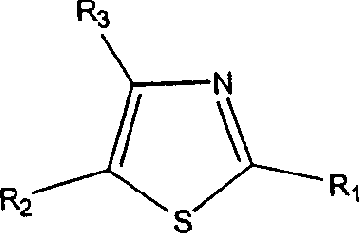
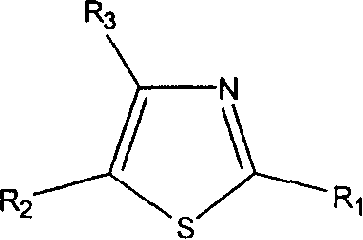
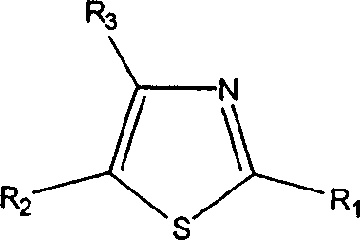
Thiazole derivatives
A drug and composition technology, applied in the direction of drug combination, metabolic disease, anti-drug, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This example describes the efficacy of the compounds of the present invention in inhibiting the formation of AGEs.
[0057] When incubated at 37°C, a control tube containing protein and sugar formed a gel with little or no residual liquid. This gel is a symbol of the large amount of cross-linking associated with saccharification. On the contrary, a treatment tube, which contains all the contents of the control tube plus the AGE inhibitor 2-amino-4,5-dimethylthiazole HCl, remains liquid, indicating 2-amino-4,5-dimethyl Thiazole HCl is an effective AGE inhibitor.
Embodiment 2
[0059] This example uses a sandwich enzyme-linked immunosorbent assay to illustrate the effect of the compound of the present invention in cleaving the cross-linked bonds associated with AGEs.
[0060]The mixture of protein and sugar is incubated to form cross-links representing AGEs and placed in a multi-well plate. The plates are then treated with 2-amino-4,5-dimethylthiazole HCl or buffer. After treatment, the plates are rinsed to remove any free protein released by the rupture of the cross-links. An antibody that reacts with the protein is then added to the plate to provide a quantitative level of protein in the plate.
[0061] The plate treated with 2-amino-4,5-dimethylthiazole HCl has less antibody bound to the plate than the control plate, indicating that 2-amino-4,5-dimethylthiazole HCl effectively splits Crosslinks related to saccharification.
Embodiment 3
[0063] This example describes the effect of the compounds of the present invention in inhibiting glucose oxidase.
[0064] In order to test the efficacy of 2-amino-4,5-dimethylthiazole HCl in inhibiting glucose oxidase, a commercially available glucose oxidase kit was used. The test showed that, compared with the control sample, in the sample containing 2-amino-4,5-dimethylthiazole HCl, H 2 O 2 The production of serotonin was reduced by more than 60%, indicating that 2-amino-4,5-dimethylthiazole HCl effectively inhibited glucose oxidase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com