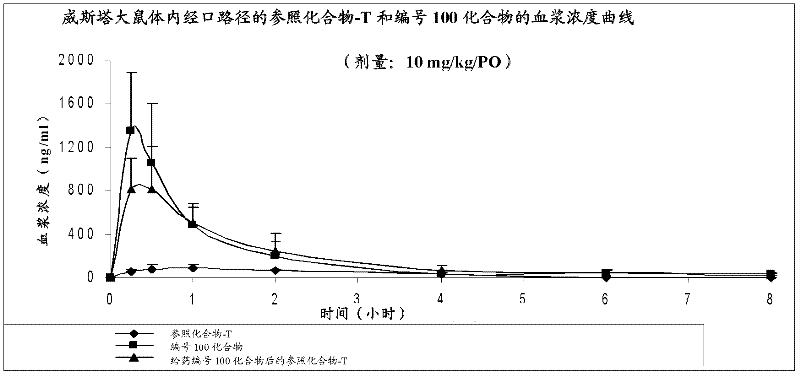
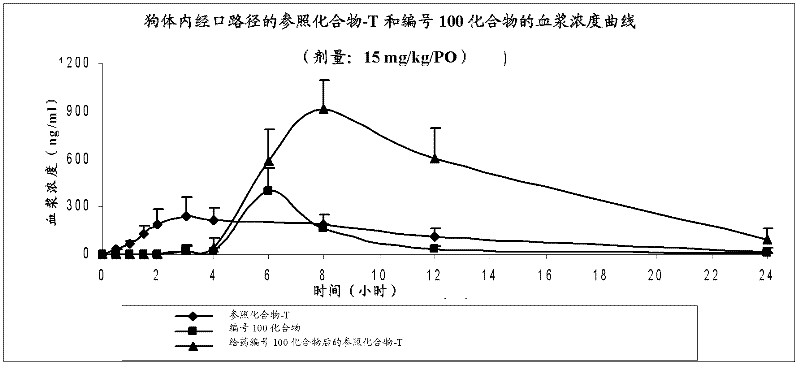
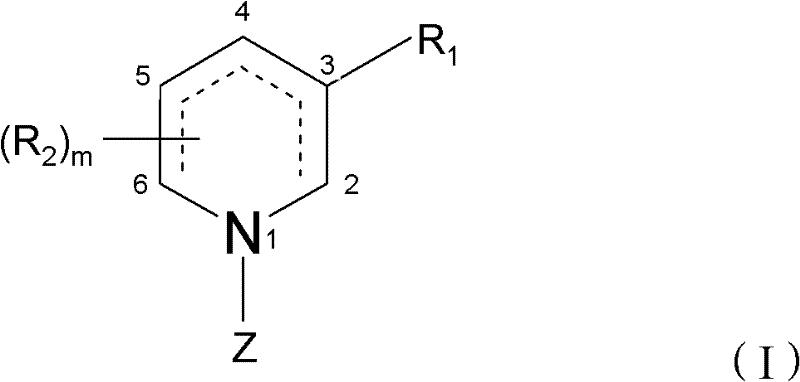
Piperidine derivatives useful for treatment of diebetes
A compound, the technology of dihydropyridine, applied in the field of diseases caused by formation and accumulation, can solve problems such as impairment of growth factor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0457] N'-(methylsulfonyl)-1-[2-oxo-2-(thien-2-yl)ethyl]-1,4-dihydropyridine-3-carbohydrazide (No. 100 compound) preparation
[0458] method 1
[0459] Into pyridine, 3-[[2-(methylsulfonyl)hydrazino]carbonyl]-1-[2-oxo-2-(2-thienyl) in batches at -10°C to 0°C under nitrogen atmosphere ) ethyl], to a stirred suspension of chloride (200 gm, 0.53 mol) in pyridine (1000 ml) was added sodium cyanoborohydride (40 gm, 0.64 mol). The resulting reaction mixture was stirred at 25-30°C for 2 hours. The solid that separated was filtered and sucked dry. The solid was dissolved in dichloromethane and washed with water. The organic layer was separated and dried over sodium sulfate and dichloromethane was evaporated. The isolated solid was filtered, washed with dichloromethane and dried under vacuum at 50-55°C to give the desired product (20 gm) as a yellow solid.
[0460] 1 H NMR (400MHz, DMSO-d 6 )
[0461] δ9.36(s, 1H), 9.12(s, 1H), 8.08-8.07(d, 1H), 8.00-7.99(d, 1H), 7.30-7.28(t, ...
example 2
[0473] Preparation of N'-(methylsulfonyl)-1-[2-oxo-2-(thien-2-yl)ethyl]-1,4-dihydropyridine-3-carbohydrazide (No. 100 compound) Alternatives to
[0474] To 1,2-dimethoxyethane (600ml) and pyridine, 3-[[2-(methylsulfonyl)hydrazino]carbonyl]-1-[2-oxo at -10°C to 0°C - To a stirred cold solution of 2-(2-thienyl)ethyl]chloride (120 gm, 0.32 mol) was added sodium cyanoborohydride (30 gm, 0.48 mol) and stirred at ambient temperature for 2-3 hours. The resulting crude product was filtered and washed with water. The crude product was stirred in water:ethanol (1:1) for 1 hour and filtered. The crude product was dried under vacuum at 55°C for 15 hours to give the desired product (61 gm).
[0475] The crude product (3 gm) was further purified by recrystallization in acetonitrile to give the title product (1.6 gm).
example 3
[0477] N'-(methylsulfonyl)-1-[2-oxo-2-(thiophen-2-yl)ethyl]-1,4,5,6-tetrahydropyridine-3-carbohydrazide (No. 183 compound) preparation
[0478] To a mixture of 10% Pd / C (1 g, 20% w / w) in methanol (25 ml) was added triethylamine (0.47 ml, 3.18 mmol) at room temperature under a nitrogen atmosphere. Subsequent partitioning of pyridine, 3-[[2-(methylsulfonyl)hydrazino]carbonyl]-1-[2-oxo-2-(2-thienyl)ethyl]chloride (1 g, 2.65 mmol) Add in batches to the above mixture. A hydrogen atmosphere (50 mbar) was applied and the reaction was continued for 50 hours at room temperature. The reaction mixture was filtered through Hyflow, the filtrate was distilled, and the residue was suspended in water. The solid obtained above was filtered and dried under vacuum. The crude product obtained above was further purified by silica gel column chromatography using ethyl acetate and hexane as eluents to give a yellow solid product (0.18 g).
[0479] 1 H NMR (400MHz, DMSO-d 6 )δ1.78(2H, s), 2.18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com