Lopinavir and ritonavir for the treatment of cervix disorders
A Lopina, Cervical technology applied in the field of cancer and disorders, which can solve the problem of low treatment outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0222] A preferred treatment regimen is described in Example 8.
[0223] Treatment of dysplasia associated with HPV
[0224] In a preferred embodiment of the invention, the pharmaceutical composition is useful for treating a female subject suffering from HPV-associated cervical dysplasia.
[0225] As used herein, "dysplasia" encompasses both preinvasive lesions and cancer. Preinvasive lesions associated with HPV include high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells of undetermined significance (ASCUS), and low-grade squamous intraepithelial lesion (LSIL). Cancers associated with HPV include, for example, cervical intraepithelial neoplasia (CIN) and invasive cervical cancer (ICC).
[0226] The disclosed methods and treatment regimens can be used to treat dysplasia associated with HPV. In some aspects, for example, the disclosed methods and treatment regimens can be used to treat HSIL. In some aspects, the disclosed methods and treatment regimens...
Embodiment 1
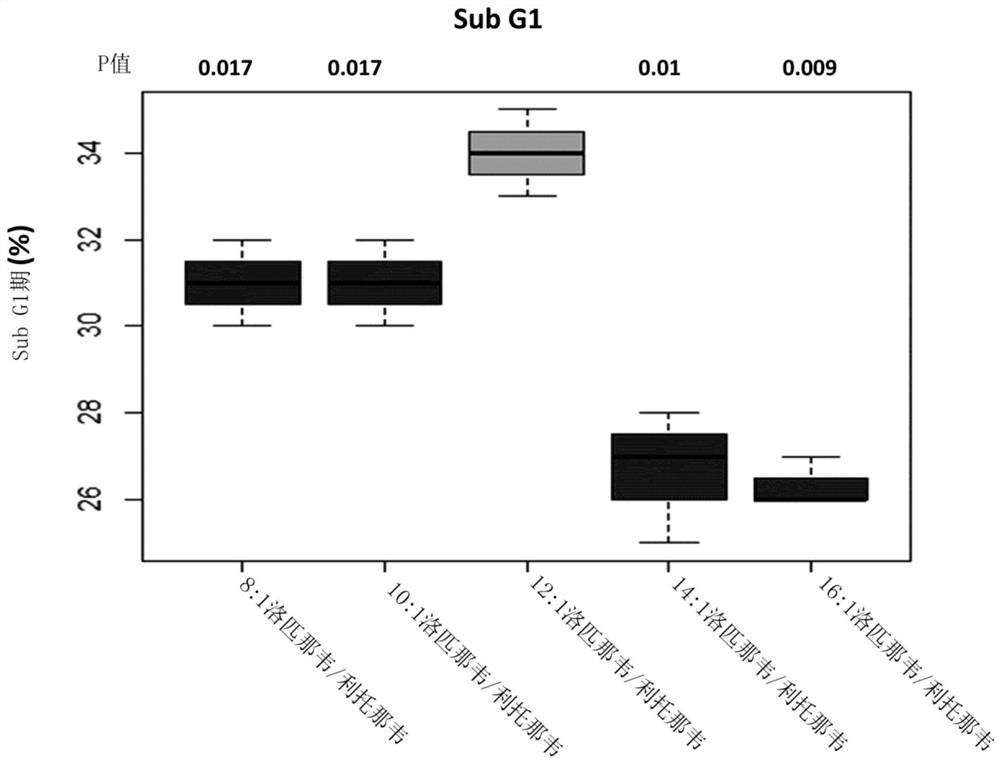
[0237] Example 1: Evaluation of the effect of lopinavir:ritonavir at a total API concentration of 20 μM in a ratio of 8:1-16:1 w / w on E6 / E7 immortalized non-transformed endocervical cells
[0238] E6 / E7 endocervical cells are a non-transformed cell line with a phenotype similar to that found in HPV-associated cervical dysplasia. Thus, the cell line represents a good model for evaluating the effects of lopinavir and ritonavir on precancerous and early cancer conditions. For example, dysplasia CIN1-CIN3 associated with HPV.
[0239] 1.1 Method
[0240] 1.1.1 Cell culture
[0241] at 5% CO 2 and 37°C, E6 / E7 cells were maintained by standard methods in RPMI growth medium containing 5% fetal calf serum (FCS).
[0242] Experiments were performed on cells inoculated from T75 confluent starter cultures into T25 flasks. The cells were cultured in T25 flasks for 6 days. Standard culture conditions were followed for the first 3 days, while on days 4-6 the cells were grown in growth...
Embodiment 2
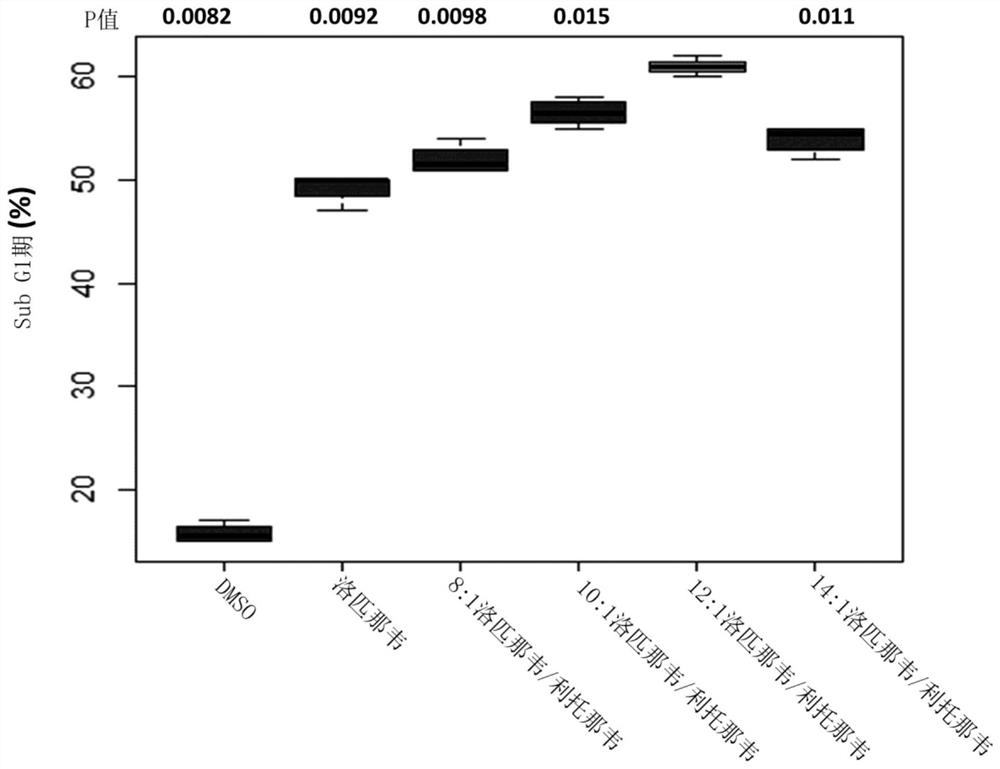
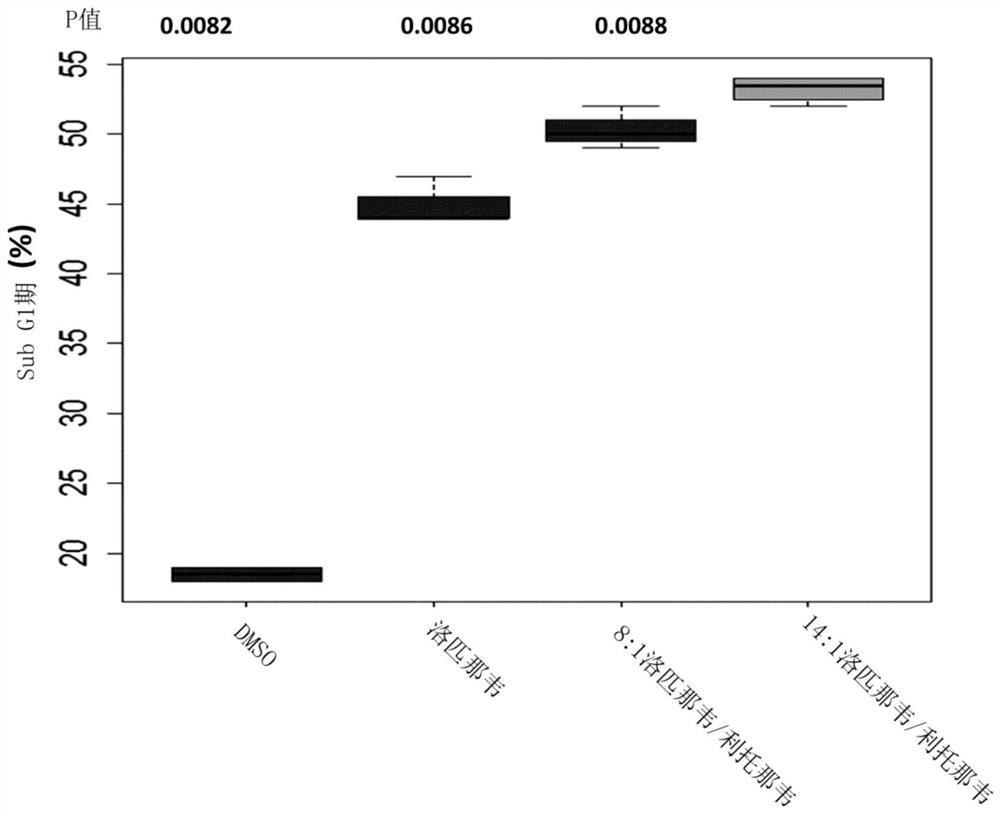
[0252] Example 2: Evaluation of the effect of lopinavir:ritonavir with a total API concentration of 20 μM in a ratio of 8:1-14:1 w / w on HPV18-positive HeLa cervical cancer cells
[0253] The effect of different ratios of lopinavir and ritonavir was evaluated in HeLa cells. HeLa cells, an HPV18-positive cervical cancer cell line, are a good model for HPV-associated malignancies such as invasive cervical cancer (ICC).
[0254] 2.1 Method
[0255] Follow the method described in 1.1, except:
[0256] 2.1.1: The cells were cultured in T25 flasks for 2 days. Standard culture conditions were followed on the first day, while the cells were grown on the second day in growth medium without FCS.
[0257] 2.1.2: Culture was continued for 2 days in RPMI growth medium containing 5% FCS and a total API concentration of 20 μΜ with lopinavir and ritonavir in the ratios stated in the results section.
[0258] 2.2 Results
[0259] Fig. 2 (A) shows with DMSO (control); 20 μ M lopinavir alone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com