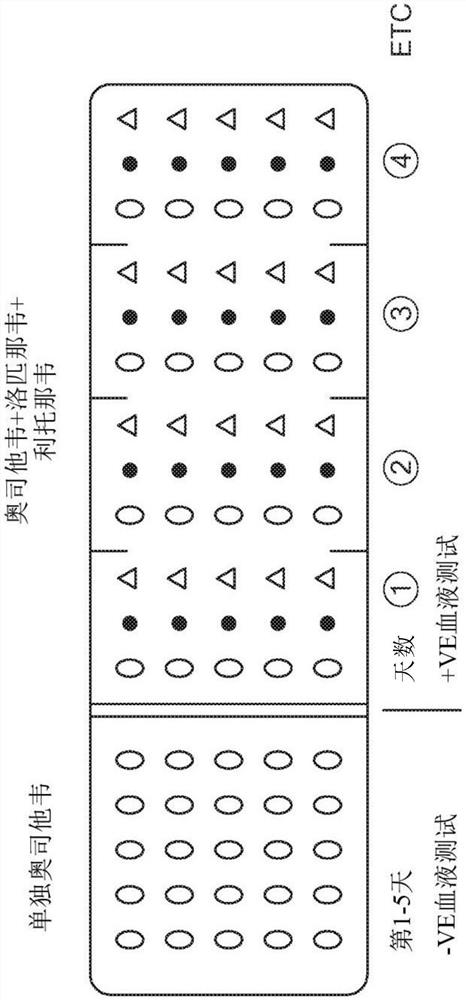
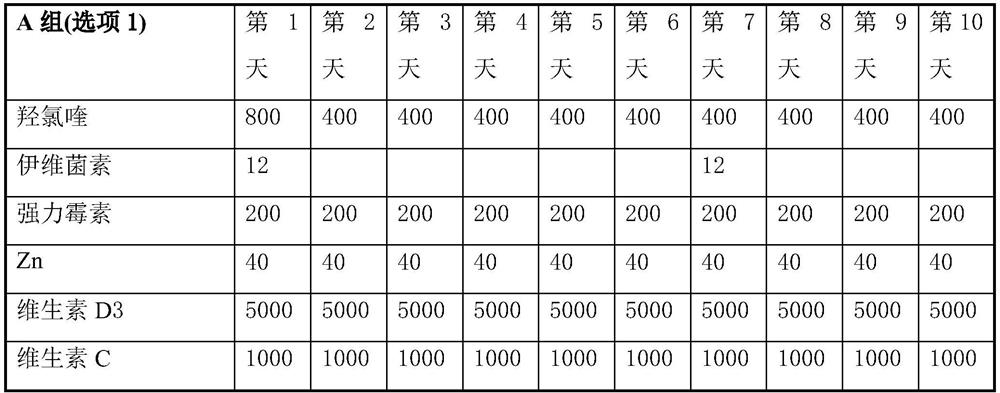
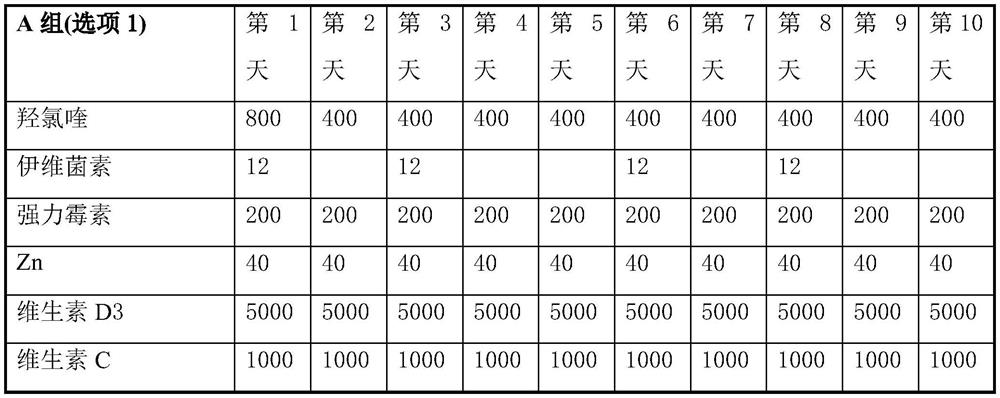
Product of manufacture and method for treating, ameliorating or preventing coronavirus infection
A product and therapeutic technology, applied in the field of infectious diseases, which can solve the problems of difficult to use antiviral agents, no single drug regimen has been found for coronavirus infection, and coronavirus anti-infective agents cannot cure infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0393] Example 1: Exemplary Treatment Protocol
[0394] A 42-year-old (y) female patient was first treated with oseltamivir (or TAMIFLU TM ) for treatment. She was treated for 4 days, but then developed fever, cough, aches and some difficulty breathing, and her blood results were positive for the COVID-19 virus.
[0395] As the patient remains positive for the 2019-nCoV virus, lopinavir and ritonavir or KALETRA are now TM , ALTERA TM 、ALUVIA TM , KALMELTREX, LOPIMUNE TM or LOPINAVIR TM Treatment was added to oseltamivir therapy, and within 48 hours, her blood test turned negative for the coronavirus.
[0396] The patient was very sick, reaching a stage of dyspnea and having trouble walking, and was expected to die if not for this new drug combination. The combination of drugs as provided herein can rapidly clear the coronavirus from a patient's blood, thereby terminating or significantly ameliorating an otherwise life-threatening disease.
Embodiment 2
[0397] Example 2: Exemplary Treatment Protocol
[0398] A 47-year-old male returning from travel presented with draggles and muscle pain and a temperature of 37.5°C. He was tested for the coronavirus and found positive for COVID-19 on a swab test. He was started on chloroquine 250 mg twice daily, lopinavir 200 mg bid, ritenovir 50 mg bid, and oseltamivir 75 mg bid (optionally, TAMIFLU TM )The combination.
[0399] His symptoms further improved within three days, and a swab test on the sixth day was negative. The muscle pain also disappeared and he felt fine and was able to be discharged home on the seventh day.
Embodiment 3
[0400] Example 3: Exemplary Treatment Protocol
[0401] A group of elderly travelers boarded a cruise ship, and many on board were exposed to the coronavirus, COVID-19, which was being tested for. This group was administered an inhalant comprising two inhalation or aerosol doses or 125 mg of chloroquine twice daily as prophylaxis / treatment. No one was infected with the COVID-19 coronavirus and tested negative for the virus when they returned home from the cruise.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com