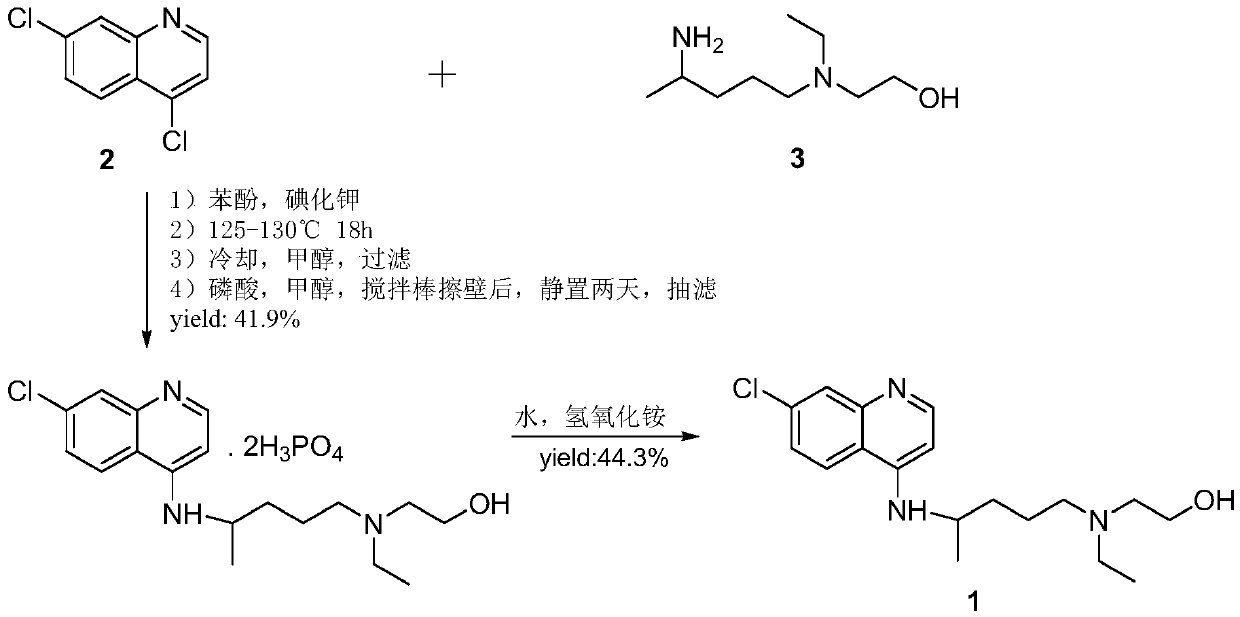
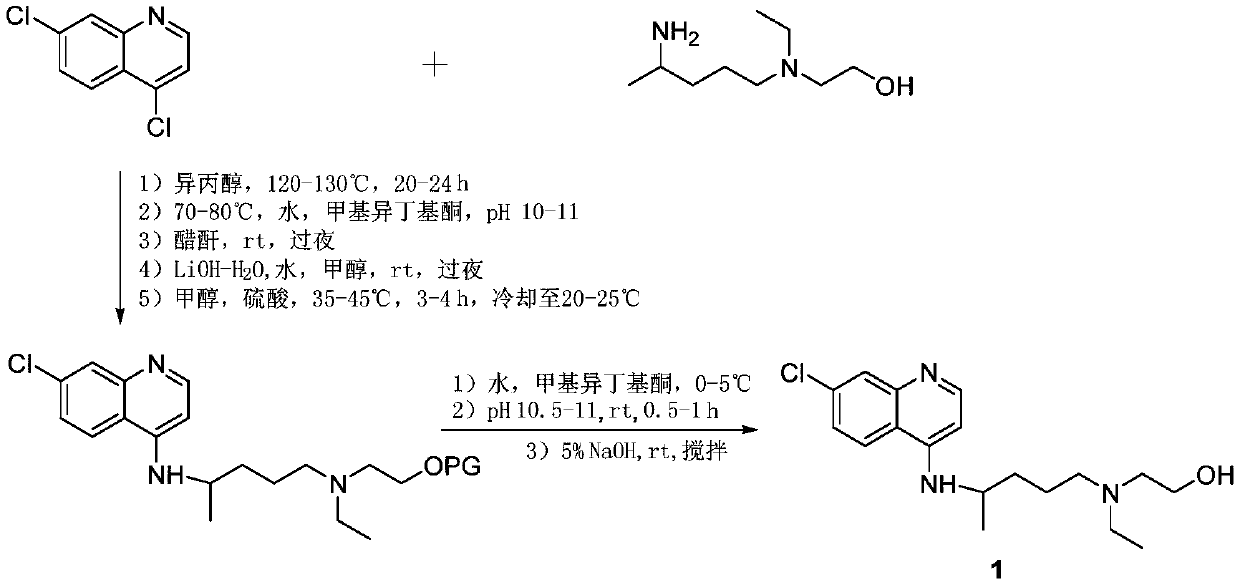
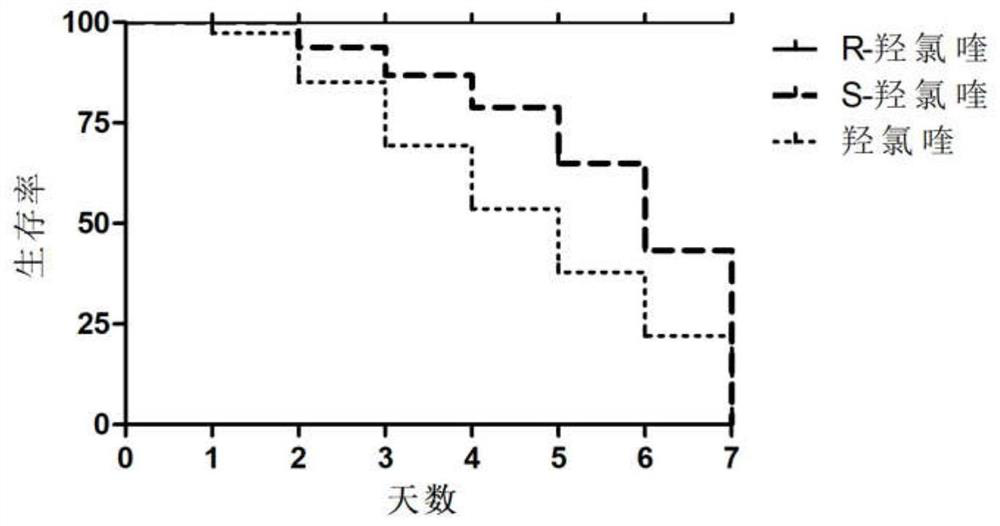
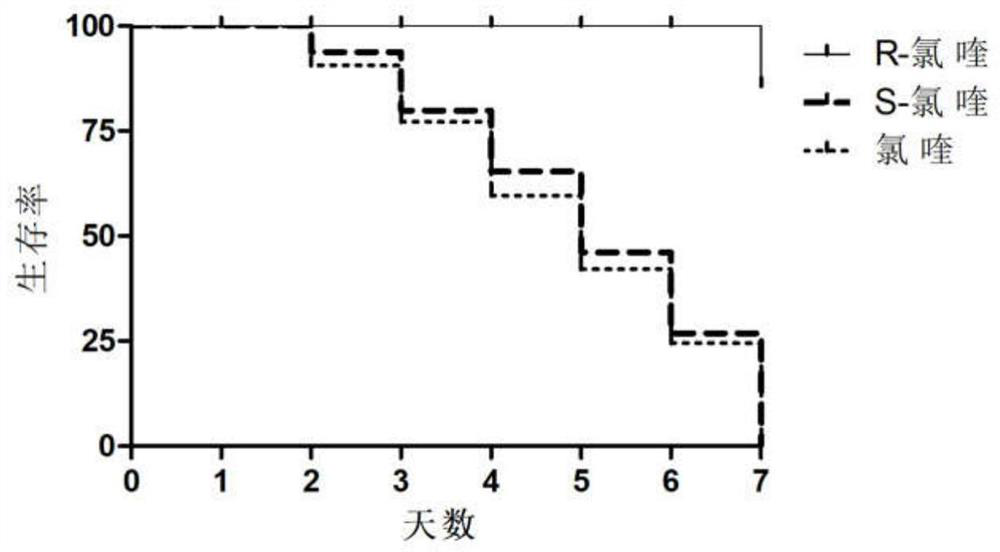
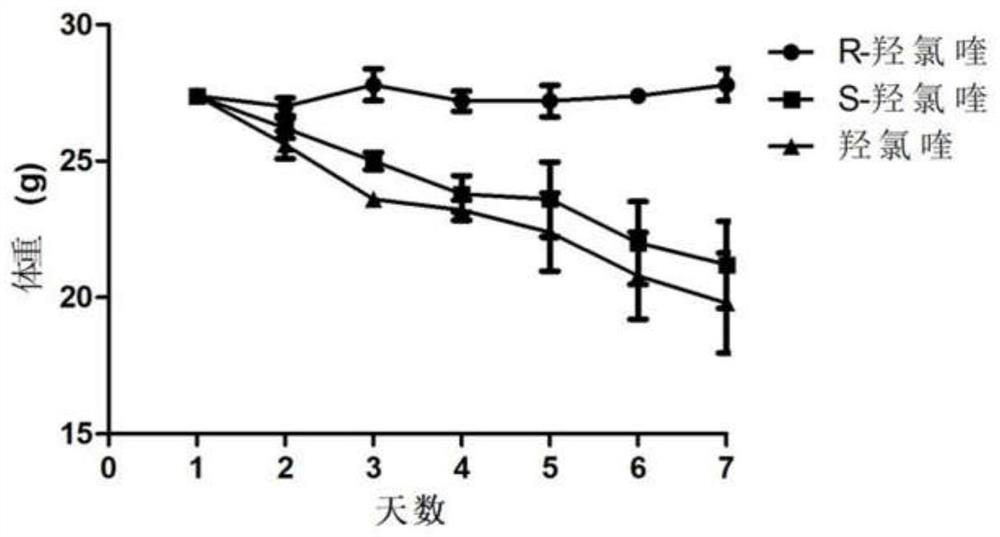
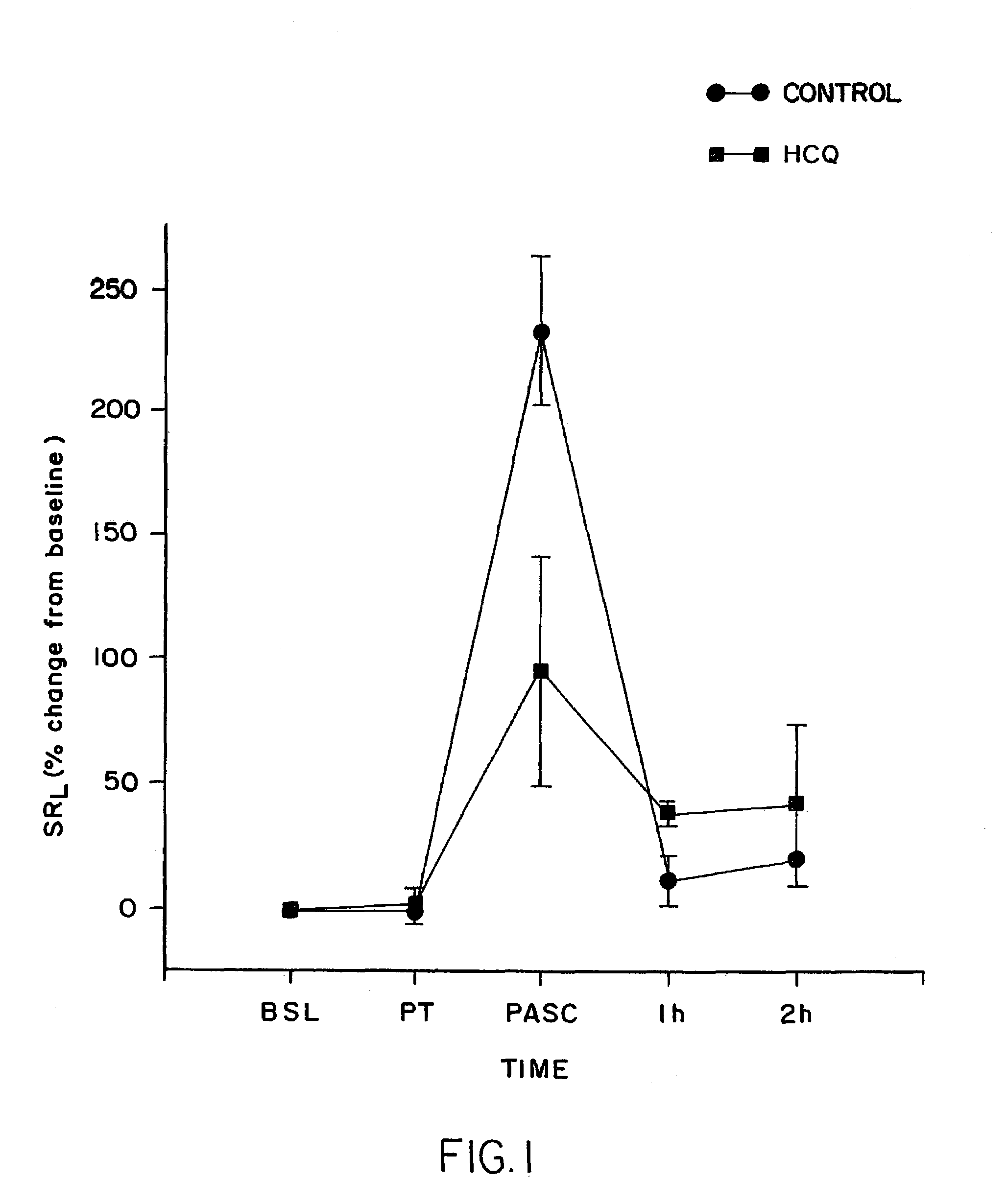
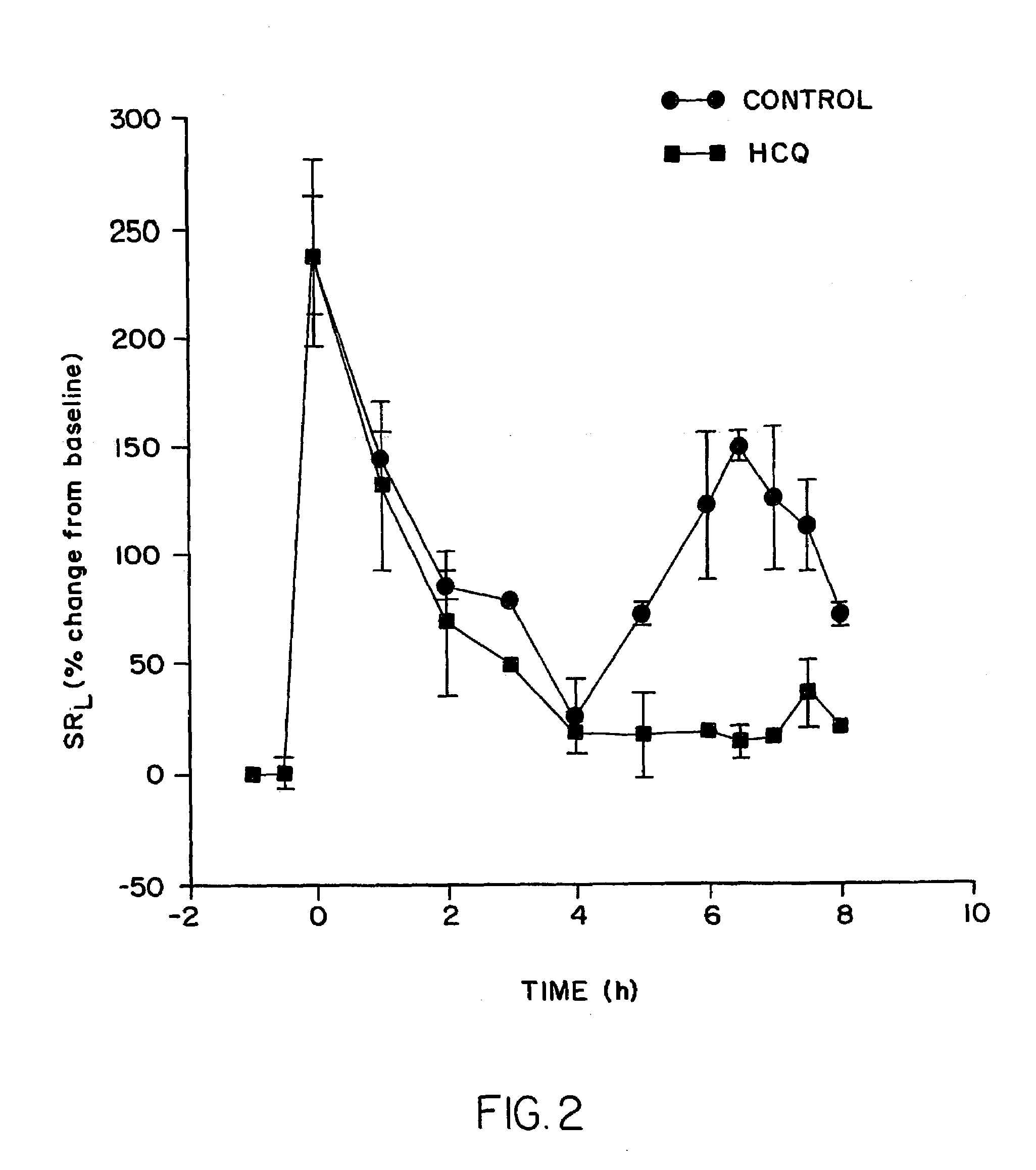
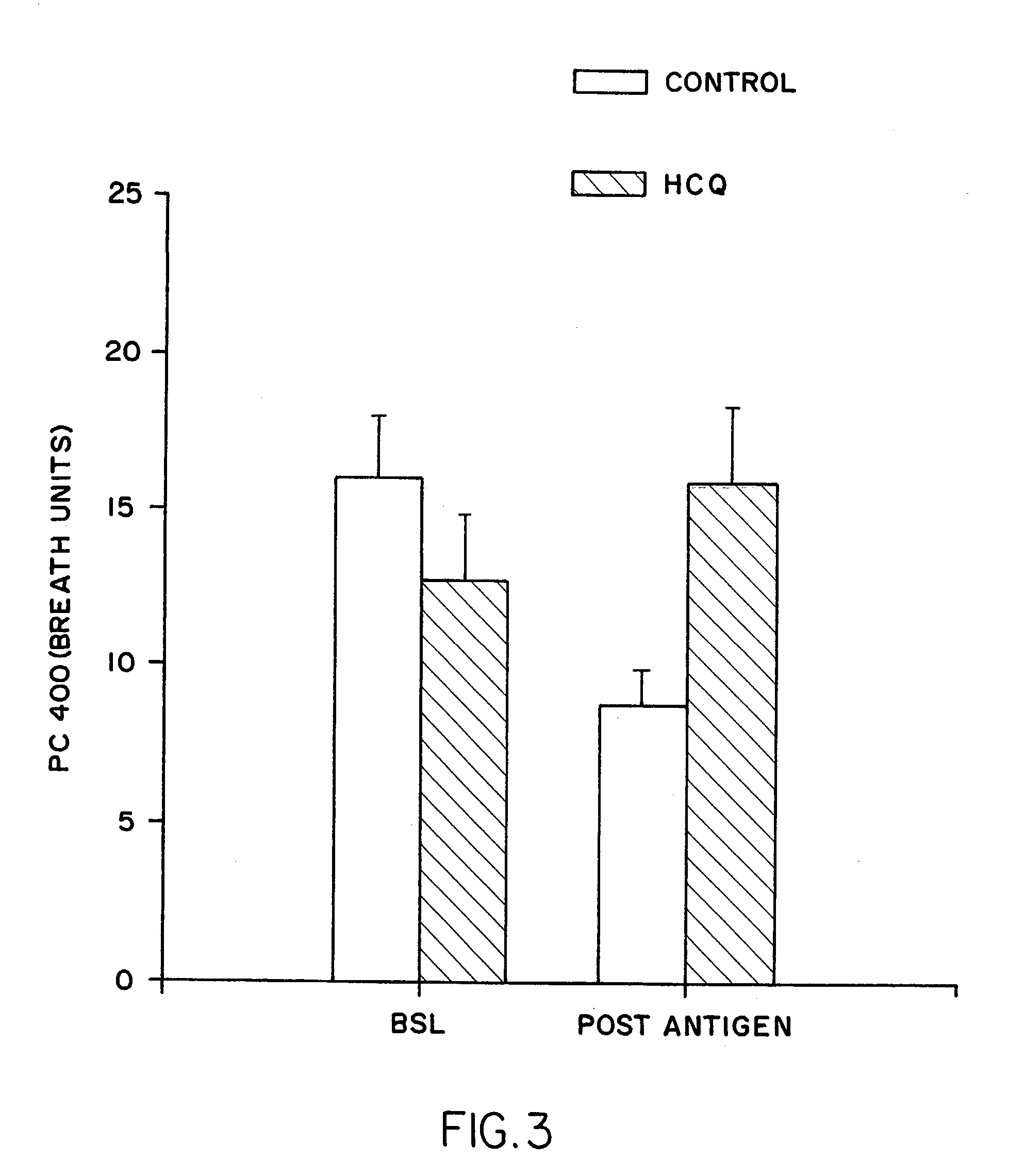
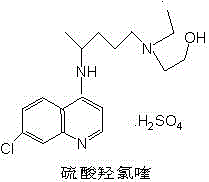
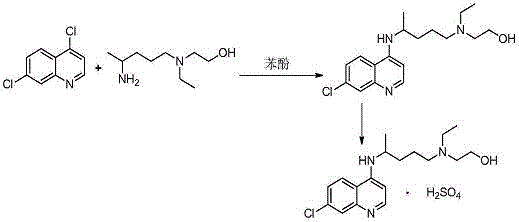
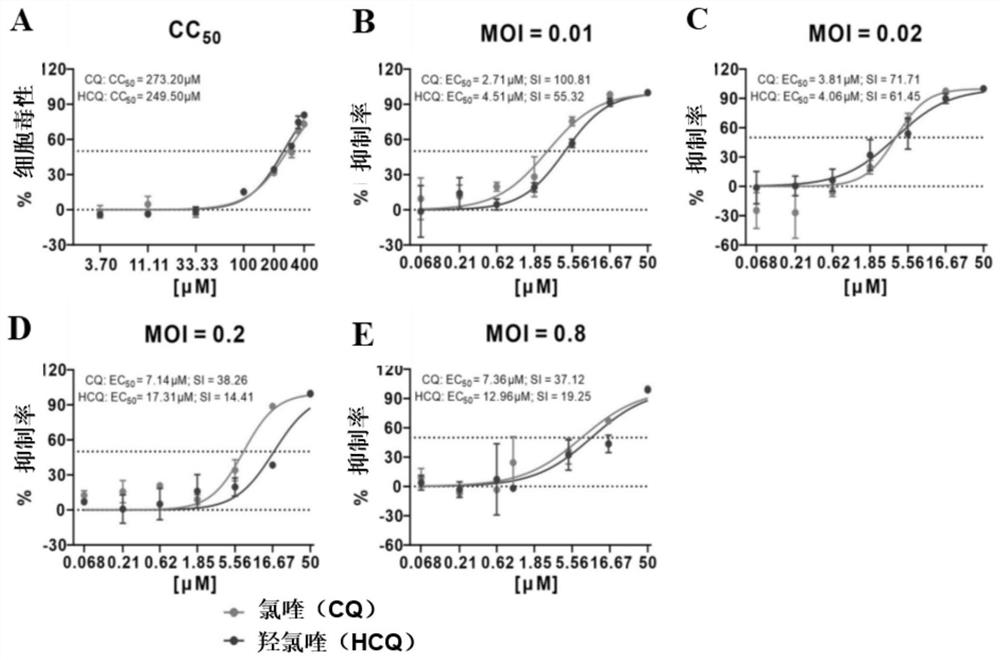
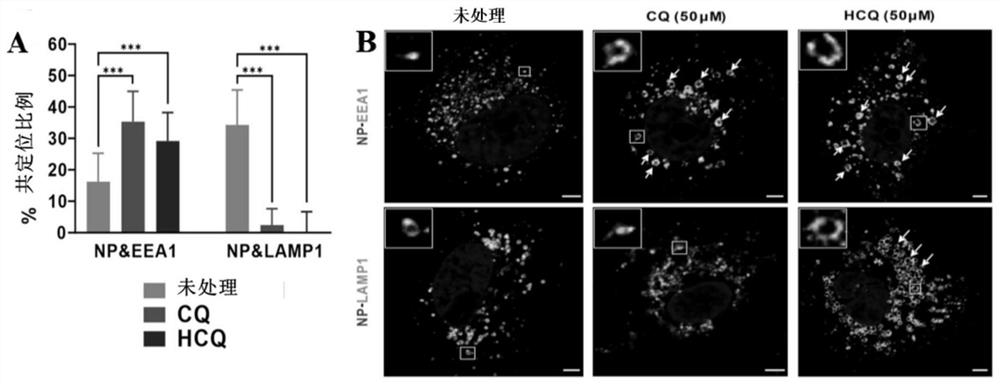
Patents
Literature
119 results about "Hydroxychloroquine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
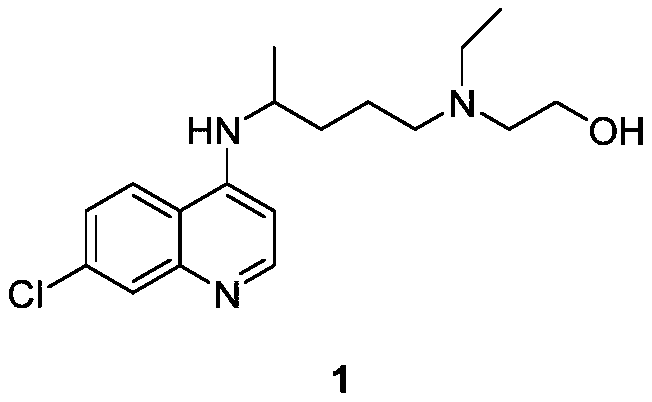
Hydroxychloroquine is used to prevent or treat malaria infections caused by mosquito bites.
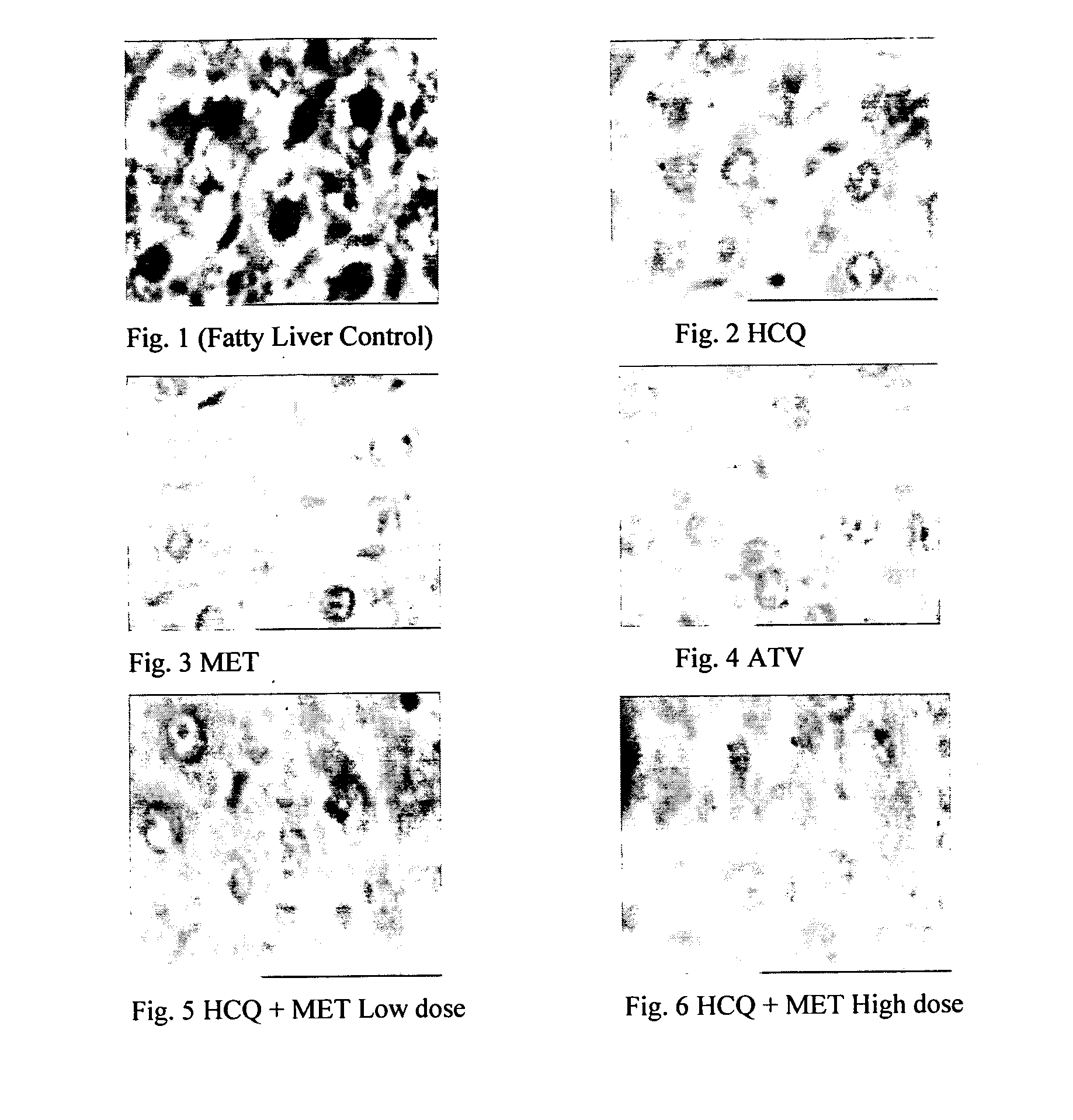
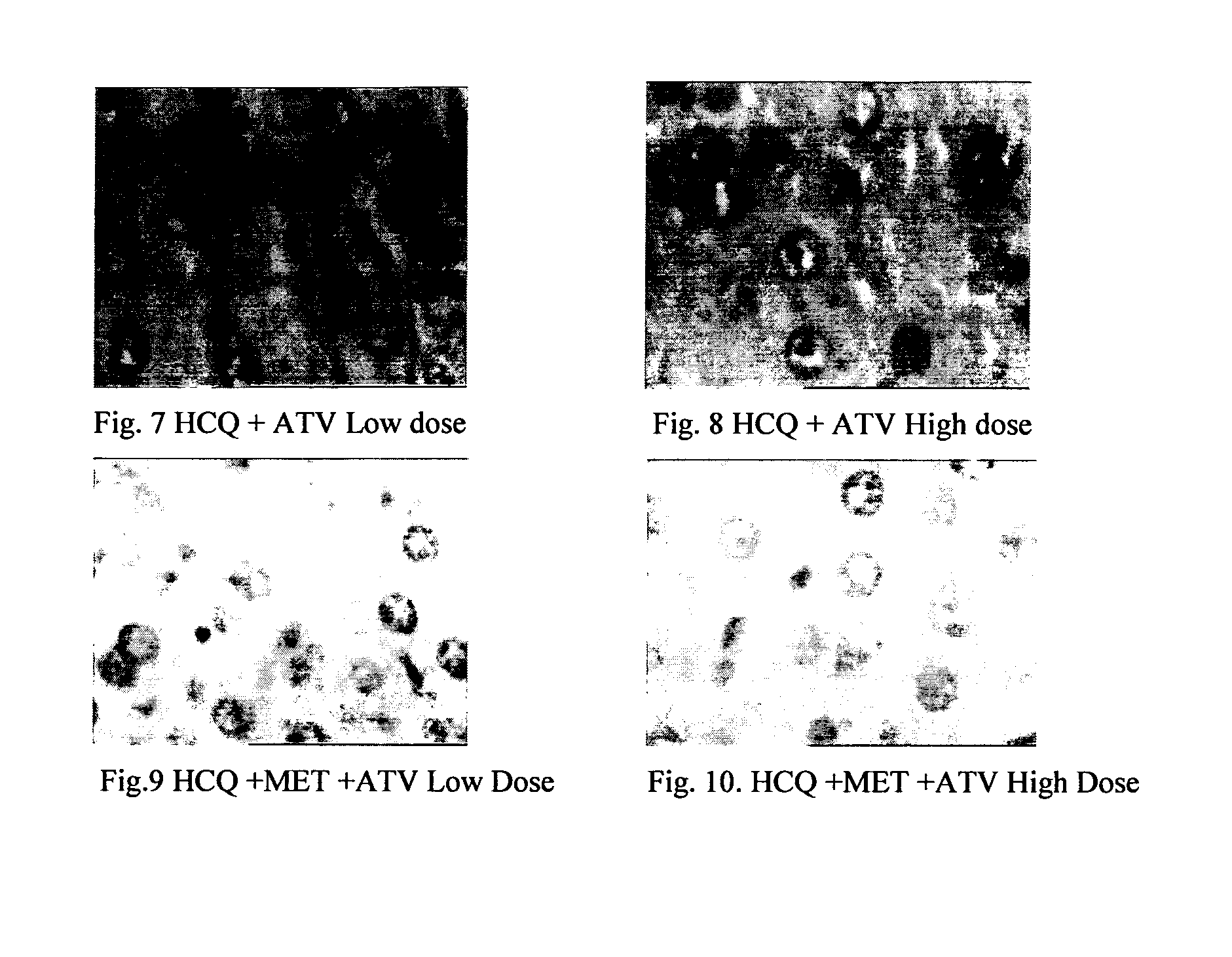
Pharmaceutical compositions for the treatment/prophylaxis of non-alcoholic fatty liver disease
InactiveUS20120202849A1Lowering/preventing accumulation of fatBiocideOrganic chemistryDiseaseHydroxychloroquine
Disclosed herein is a novel synergistic pharmaceutical composition comprising hydroxychloroquine with insulin sensitizing agents and lipid lowering agents such as statins along with pharmaceutical excipients / carriers useful in treating Non-Alcoholic Fatty Liver Disease.
Owner:IPCA LAB LTD
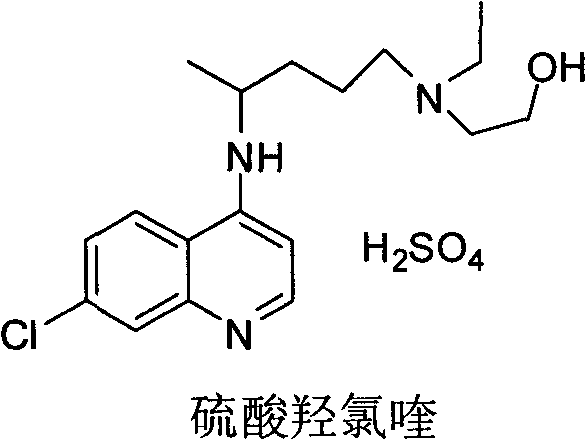
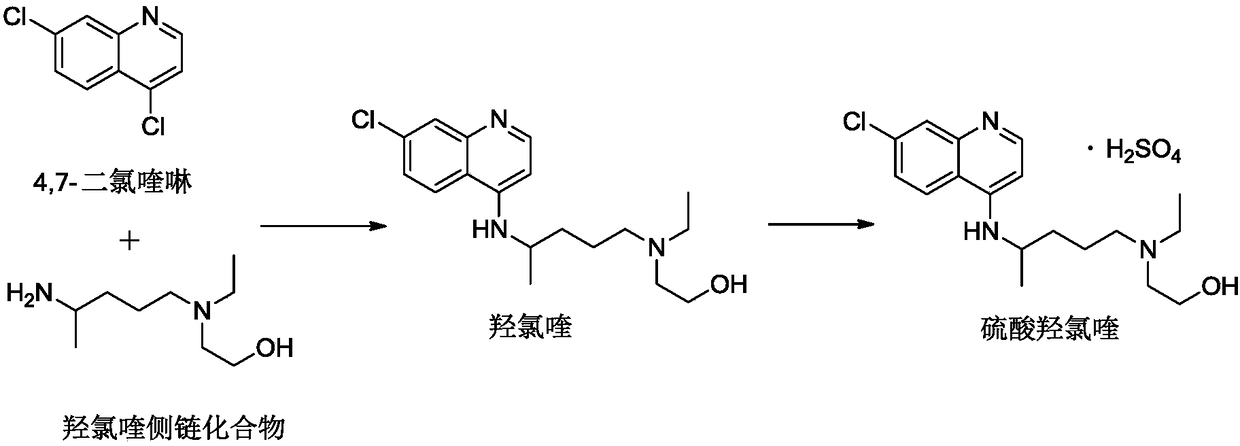
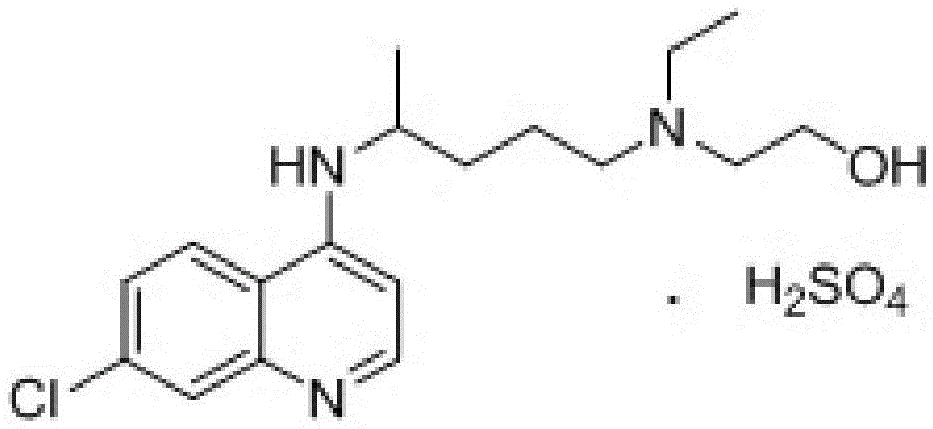
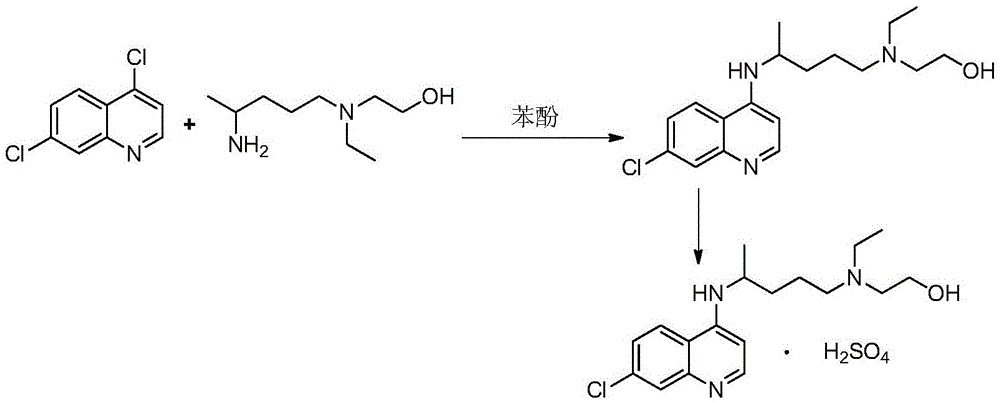
Industrial preparation method of hydroxychloroquine sulfate
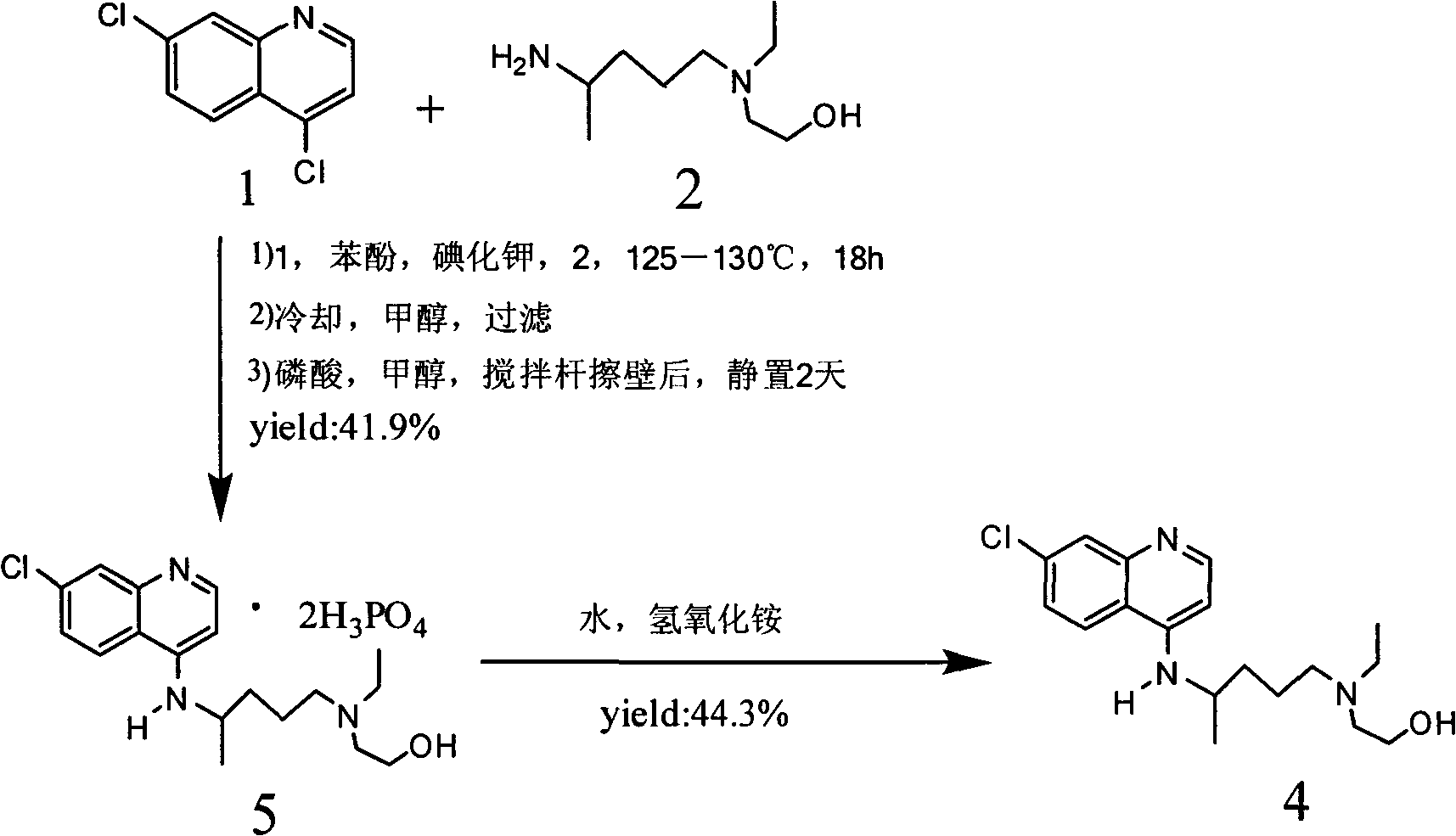
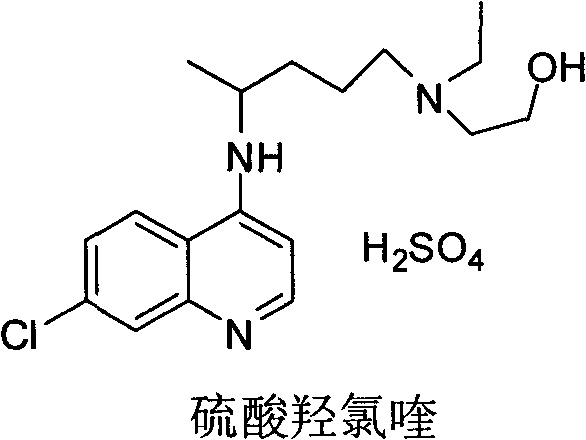
The invention relates to an industrial preparation method of hydroxychloroquine sulfate, which comprises heating 4, 7-dichloroquinoline and hydroxychloroquine side chain at refluxing temperature to 120-125 DEG C, allowing reaction to obtain hydroxychloroquine, and reacting with sulfuric acid to obtain hydroxychloroquine sulfate. The method can obtain high-purity hydroxychloroquine sulfate with single impurity less than or equal to 0.1% and purity higher than or equal to 99.5%; and has less preparation procedures, simple process, high product yield, good quality, low environmental pollution, no use of highly toxic solvent, and is easy for industrial production.
Owner:CHONGQING KANGLE PHARMA
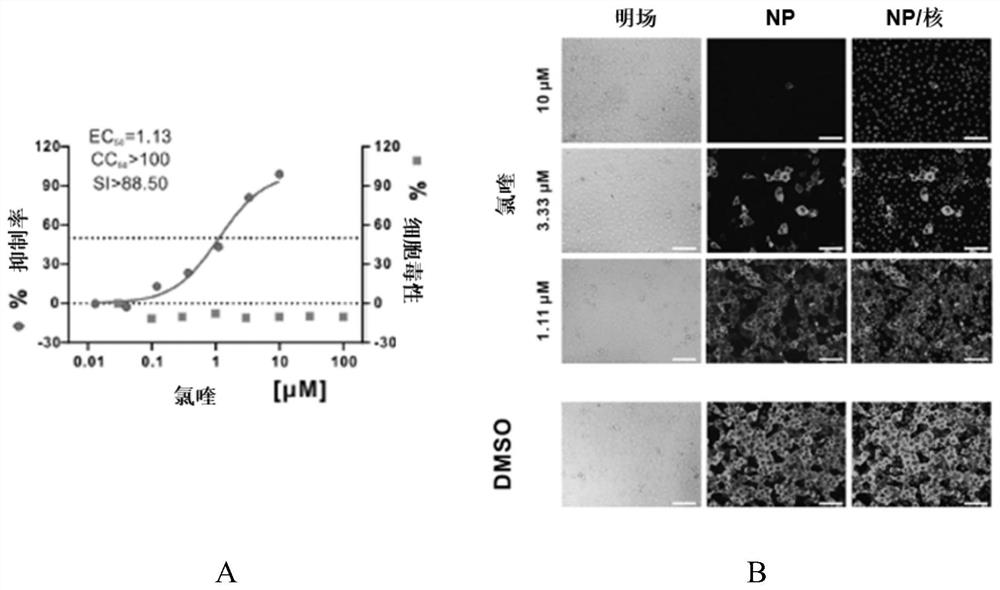
Preparation method and application of indocyanine green loaded self-assembled multifunctional nano targeting system
InactiveCN105193831AEffective treatmentThe synthesis process is simpleOrganic active ingredientsEnergy modified materialsHydroxychloroquineSide effect
The invention relates to a preparation method and application of an indocyanine green loaded self-assembled multifunctional nano targeting system. The indocyanine green loaded self-assembled multifunctional nano targeting system can be used for effectively solving the problems of the existing antitumor drugs in tumor therapy that the targeting property is poor, the toxic or side effects are high, the half decay time is short, the dosage of administration is high, the function is single, and the like. Doxorubicin is connected with hydroxychloroquine in a manner of using disulfide bonds as connecting arms so as to form self-assembled nanoparticles, then, indocyanine green is physically adsorbed into the nanoparticles, and then, the nanoparticles are wrapped by phospholipid polyethylene glycol folic acid so as to form the indocyanine green loaded self-assembled multifunctional nano targeting system, wherein each disulfide bond is 3,3'-dithiodipropionic acid or 2,2'-dithio diacetic acid, and the molecular weight of phospholipid polyethylene glycol folic acid is 1-4kDa. The indocyanine green loaded self-assembled multifunctional nano targeting system is simple and convenient in synthesis process, has the advantages of good biocompatibility, high targeting property, reduction sensitivity, hypotoxicity and the like and is an innovation of pharmaceutical preparations for the diagnosis and treatment of tumors.
Owner:ZHENGZHOU UNIV
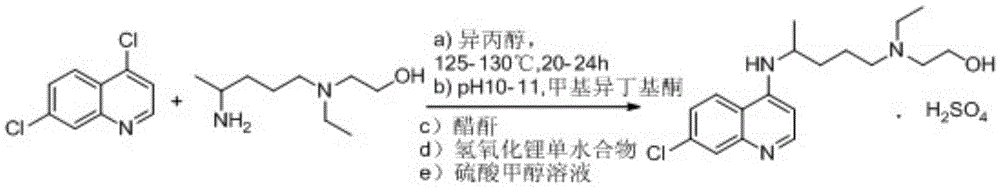
Novel industrial production method for hydroxychloroquine sulfate
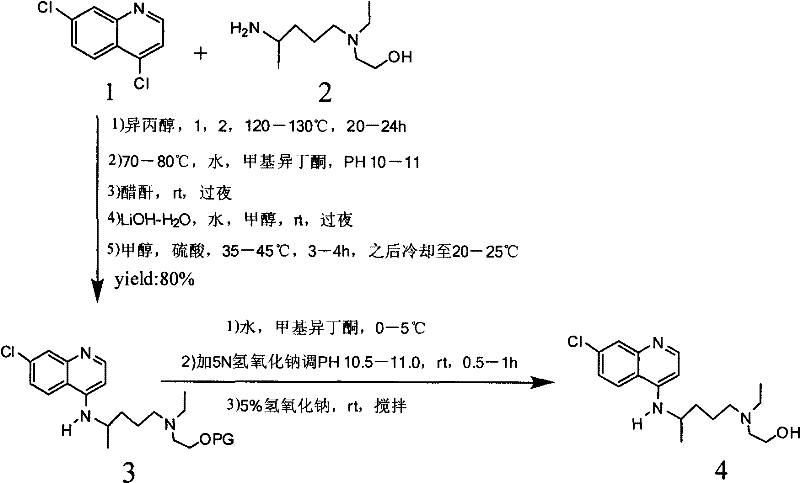
The invention provides an industrial production method for hydroxychloroquine sulfate, which includes the following steps: enabling 4.7-dichloroquinoxaline and 5-(N-ethyl-N-ethoxyl)-2-amino pentane to react under gas shield for 13-24 h at a gradually increased temperature of 120-130 DEG C to obtain hydroxychloroquine; preparing the hydroxychloroquine sulfate after the reaction between the hydroxychloroquine and an alcohol sulfate solution at the temperature of 20-30 DEG C. According to the method, the yield of the obtained crude product of the hydroxychloroquine is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate HPLC is not smaller than 99.5%, the yield of single impurity is not larger than 0.1%, so that requirements of United States Pharmacopeia is met; the novel method is simple in procedure, is environment-friendly and easy in industrial production.
Owner:WUHAN WUYAO PHARMA
New use of anti-impaludism medicament hydroxyl chloroquine
InactiveCN101428025AMinimize or control damageClear anti-inflammatory activityOrganic active ingredientsAntineoplastic agentsHydroxychloroquineLysosome
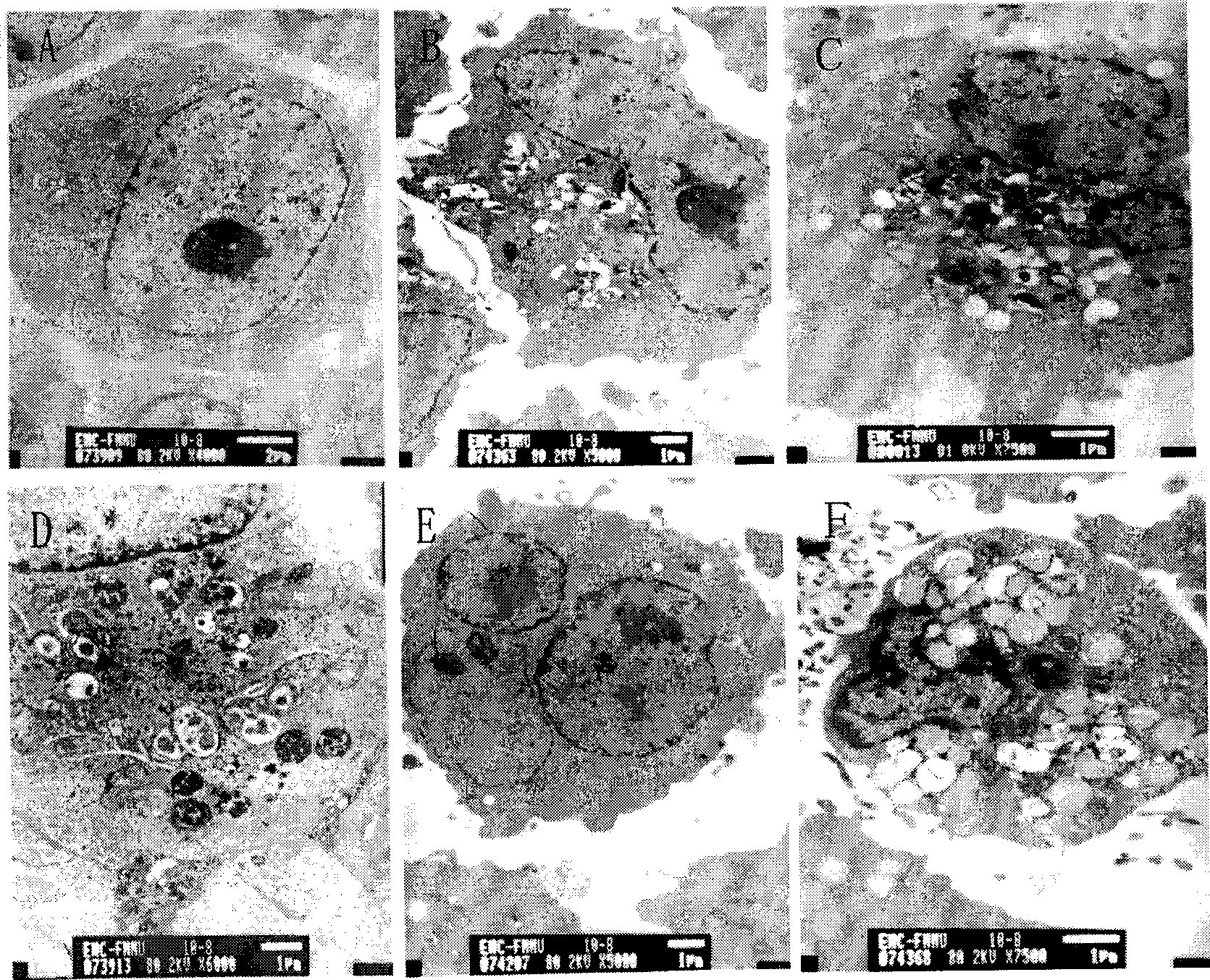
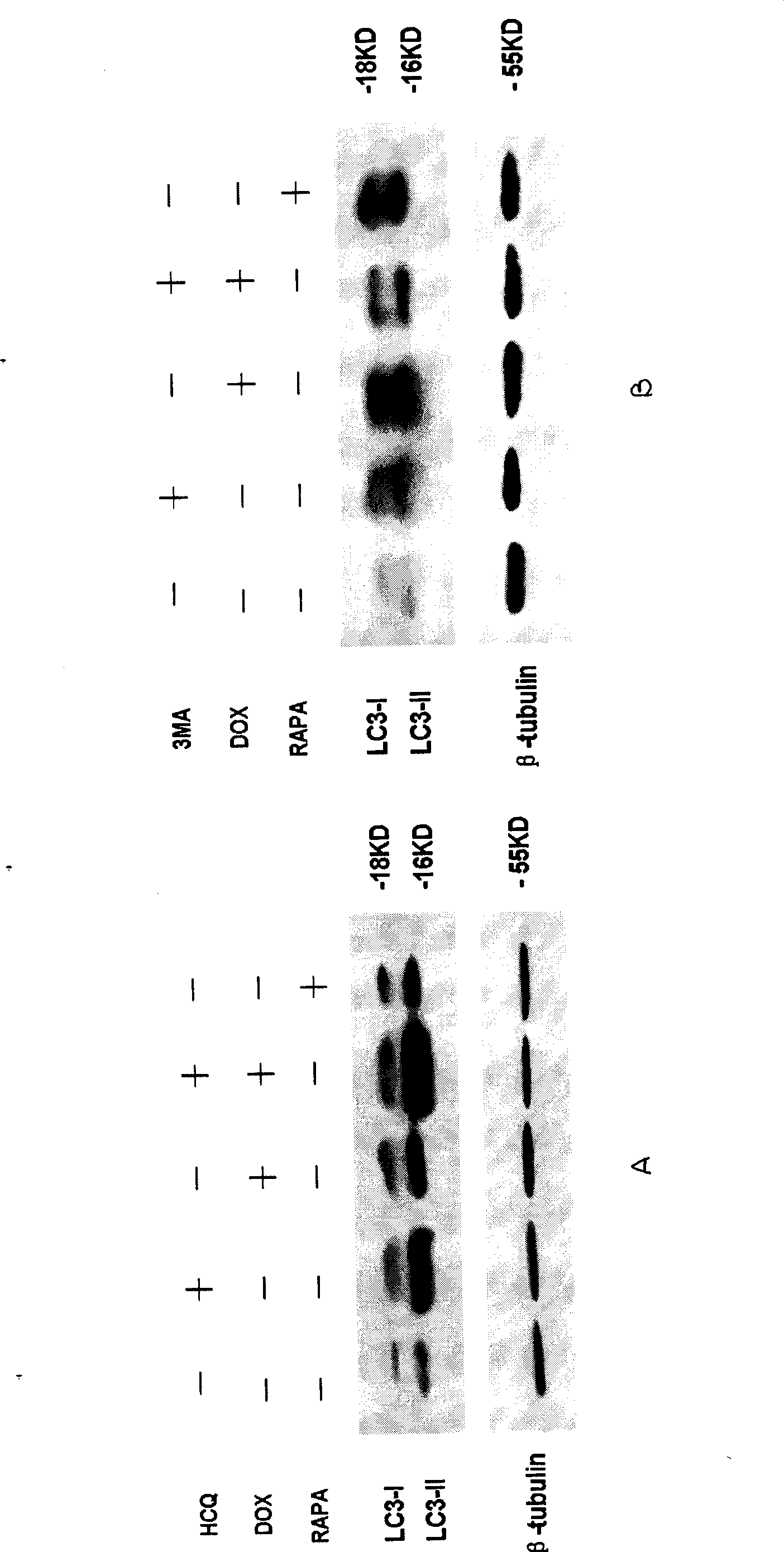
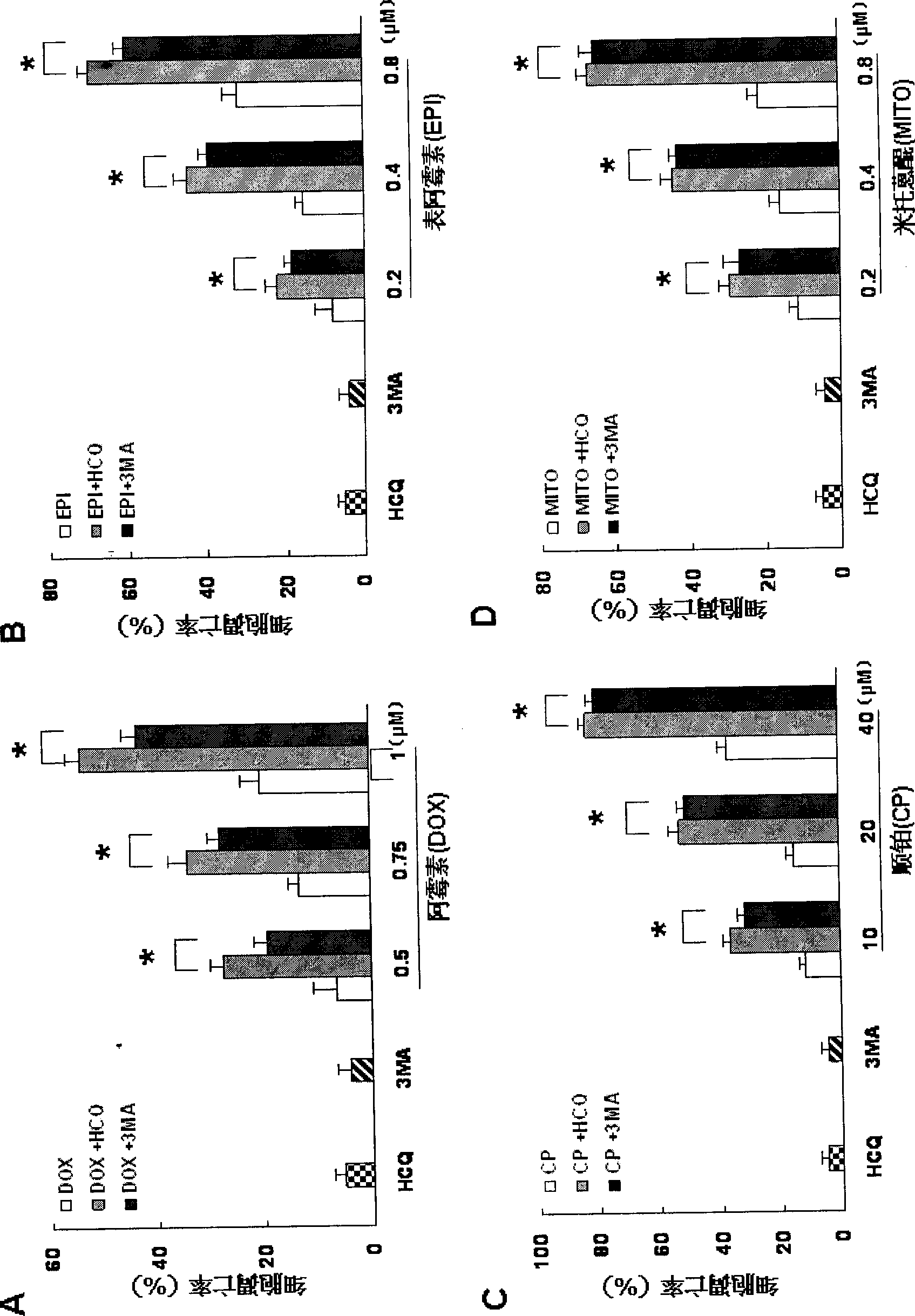
The invention relates to the novel usage of an antimalarial medicine, in particular to the novel usage of hydroxychloroquine having sensitivity enhancing effect on various chemical anti-multiple myeloma medicaments. The novel usage is characterized in the application of hydroxychloroquine in treatment of multiple myeloma through chemotherapeutic medicaments. The hydroxychloroquine can obviously enhance the killing activity of chemotherapeutic medicines such as doxorubicine, epidoxorubicin, cis-platinum and mitoxantrone to multiple myeloma cells, and the killing activity thereof is realized by inhibiting the myeloma cell autophagy signals by hydroxychloroquine. Hydroxychloroquine can induce the quantity of the autophagy lysosomes of the multiple myeloma cells to be increased and the expression of the autophagy-associated albumen LC3-II to be increased.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
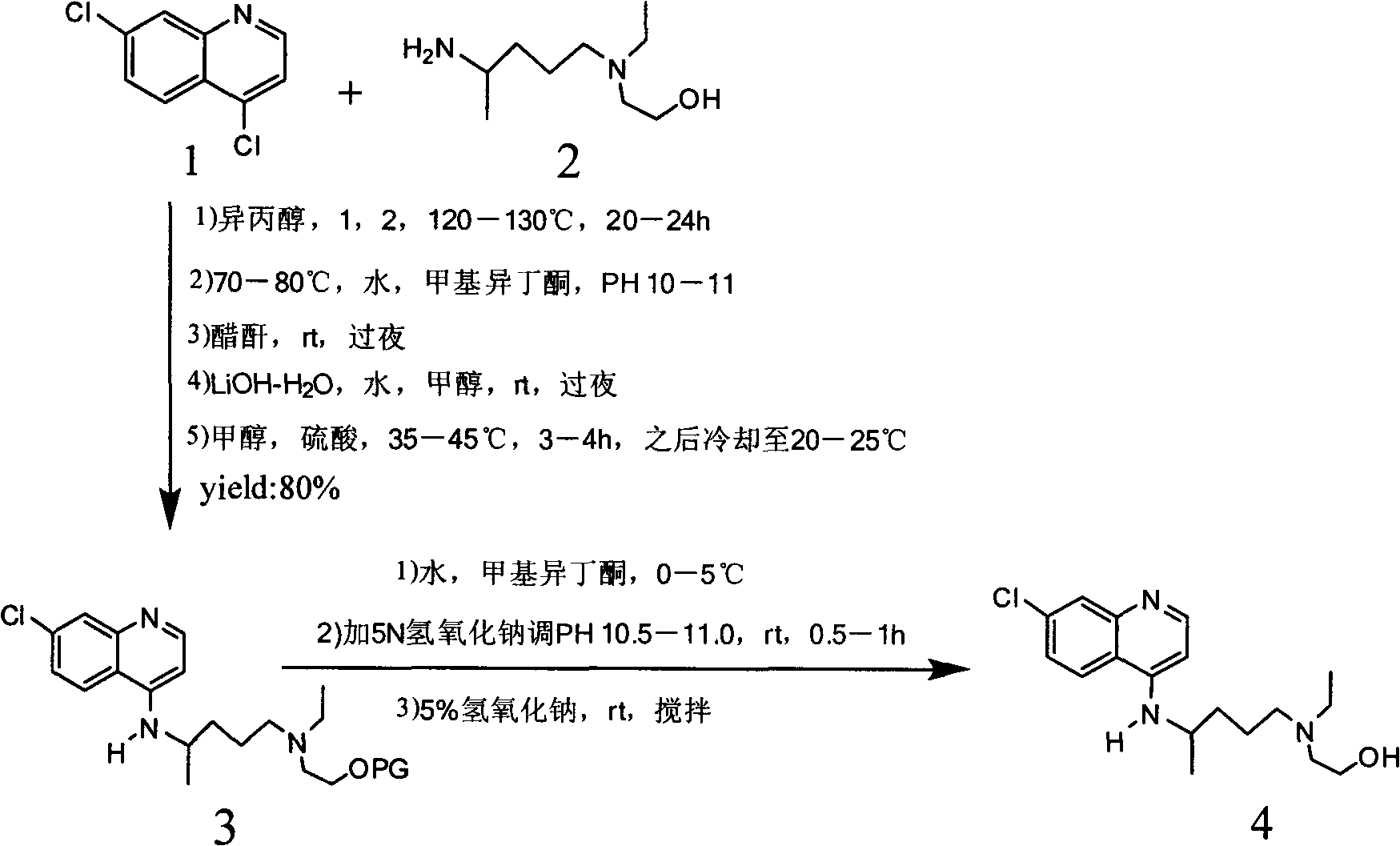
Hydroxychloroquine synthetic method
InactiveCN110283121AEasy to recycleReduce manufacturing costOrganic chemistryHydroxychloroquineAfter treatment
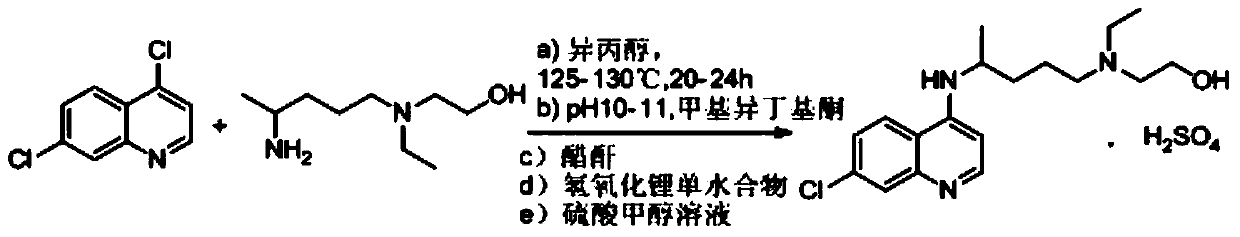
The invention provides a hydroxychloroquine synthetic method, including the steps of mixing 4,7-dichloroquinoline, 2-[(4-aminopentyl)(ethyl)amino]ethanol and N,N-diisopropylethylamine, reacting under protective gas, and after the reaction, performing extraction, concentration and purification to obtain the hydroxychloroquine. By using the synthetic method provided by the invention, N,N-diisopropylethylamine is used as both an acid-binding and a solvent to promote smooth reaction, the amount is small (only theoretical amount), and the consumption is low; the reaction time is short, alkalization is not needed after treatment, the hydroxychloroquine can be obtained by just the operations of extraction and recrystallization, and the operation is simple; the extraction solvent and the recrystallization solvent may be the same solvent, which is beneficial to the recovery and utilization of the solvent, and the production cost is reduced; the total recovery is increased from 45.9% to 74.7%, the product quality is increased from 99.0% to 99.8% or above (HPLC purity), and single impurity being less than or equal to 0.1%.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Preparation method of hydroxychloroquine and sulfate thereof
The invention discloses a preparation method of hydroxychloroquine and sulfate thereof. The preparation method of the hydroxychloroquine comprises the following steps of step (1), under the inert gasprotection atmosphere, enabling 4,7-dichloroquine and hydroxychloroquine side chain compounds to react at the temperature of 134 to 144 DEG C until the content of 4,7-dichloroquine is smaller than orequal to 10%, so as to obtain a crude product of the hydroxychloroquine, wherein the content of the hydroxychloroquine in the crude product of the hydroxychloroquine is greater than 92%; step (2), recrystallizing the obtained crude product of the hydroxychloroquine in step (1) in a mixed solvent of alcohol solvent and ester solvent, so as to obtain a refined product of the hydroxychloroquine. Thepurity of the refined product of the hydroxychloroquine can reach 99.9%, the maximum content of single impurity is controlled within 0.06%, and the total content of other impurities is smaller than 0.04%.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
Compositions and methods for treating and inhibiting viral infections
InactiveCN104703601AInfection fromReduce the risk of transmissionOrganic active ingredientsAntiviralsInfected cellHydroxychloroquine
Compositions and methods for the treatment, as well as the inhibition and prevention, of an infection of the papillomavirus and the epithelial lesions, namely, the waits of the skin and mucosal surfaces, associated therewith, in a mammalian host, as well as methods of inhibiting the replication of a papillomavirus in an infected cell, are provided. The compositions comprise a therapeutically effective amount of an active ingredient comprising at least one compound selected from the group consisting of chloroquine, hydroxychloroquine, amodiaquine, or in each case, a pharmaceutically acceptable salt thereof. The methods comprise topically administering a therapeutically and / or antivirally effective amount of such a compound to a mammalian host, such as a human being, in need of such treatment, although alternatively other routes of administration may be used, including but not limited to transdermal, transmucosal, respiratory, and by injection. The compositions optionally also comprise one or more pharmaceutically acceptable non-active ingredients.
Owner:GRACELAND BIOTECH +1
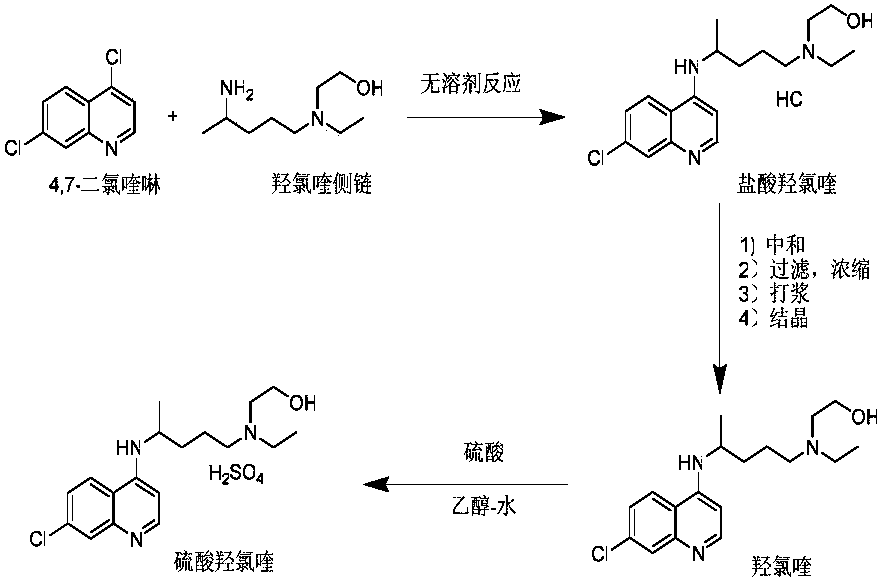
Preparation method of hydroxychloroquine sulfate
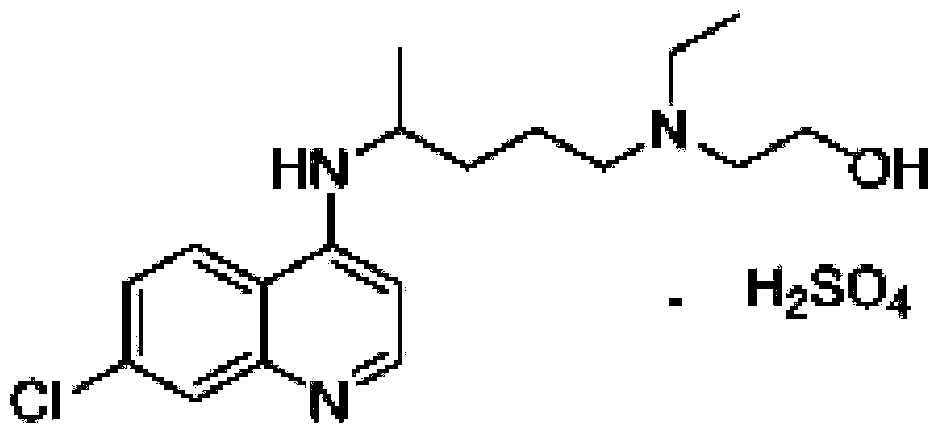
The invention discloses a preparation method of high-purity hydroxychloroquine sulfate. The method uses 4,7-dichloroquinoline and hydroxychloroquine side chain as raw materials to directly prepare hydroxychloroquine hydrochloride, hydroxychloroquine hydrochloride is neutralized with sodium alcoholate or potassium alcoholate, filtered, concentrated, beaten and crystallized to obtain hydroxychloroquine refined product, and the hydroxychloroquine refined product finally is subjected to salifying reaction with sulfuric acid in a certain proportion of pure aqueous solution to obtain hydroxychloroquine sulfate. The method avoids the use of phenol or its catalyst in the process of preparing hydroxychloroquine, avoids the extraction operation in the post-treatment, has high product purity, and basically does not generate waste water in the production process. The method is convenient to operate and has high yield, the HPLC purity of prepared hydroxychloroquine sulfate is more than or equal to99.6%, and maximum single impurity is less than or equal to 0.1%, and that method is more suitable for industrial production.
Owner:SHANGHAI INST OF TECH
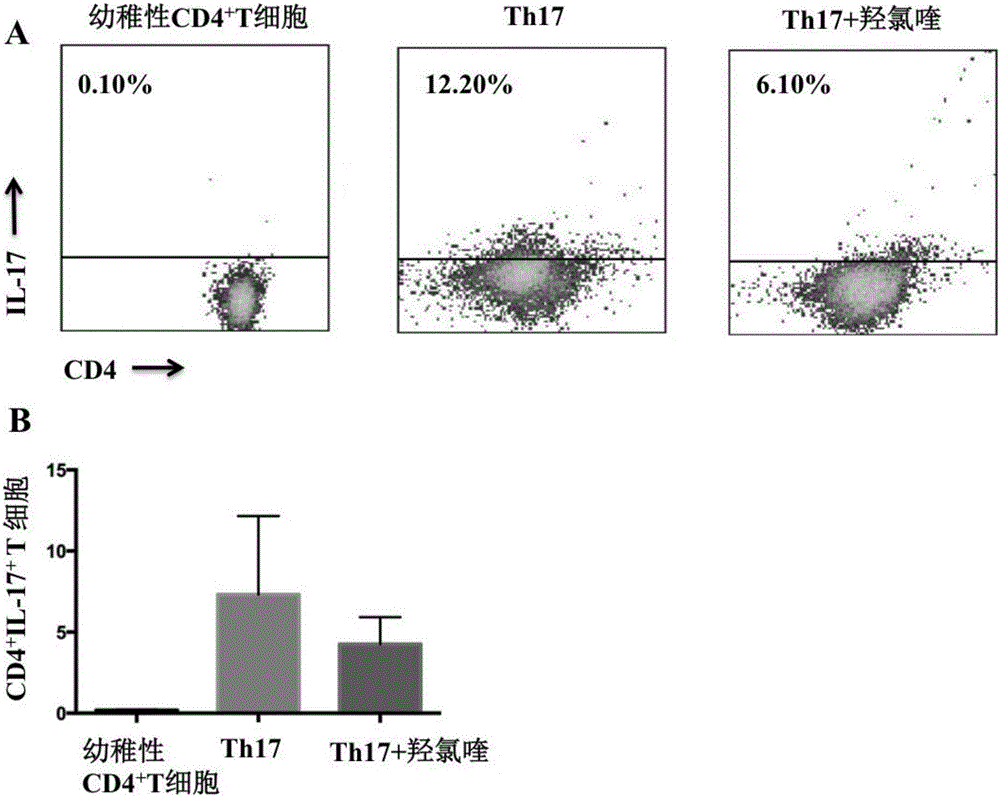
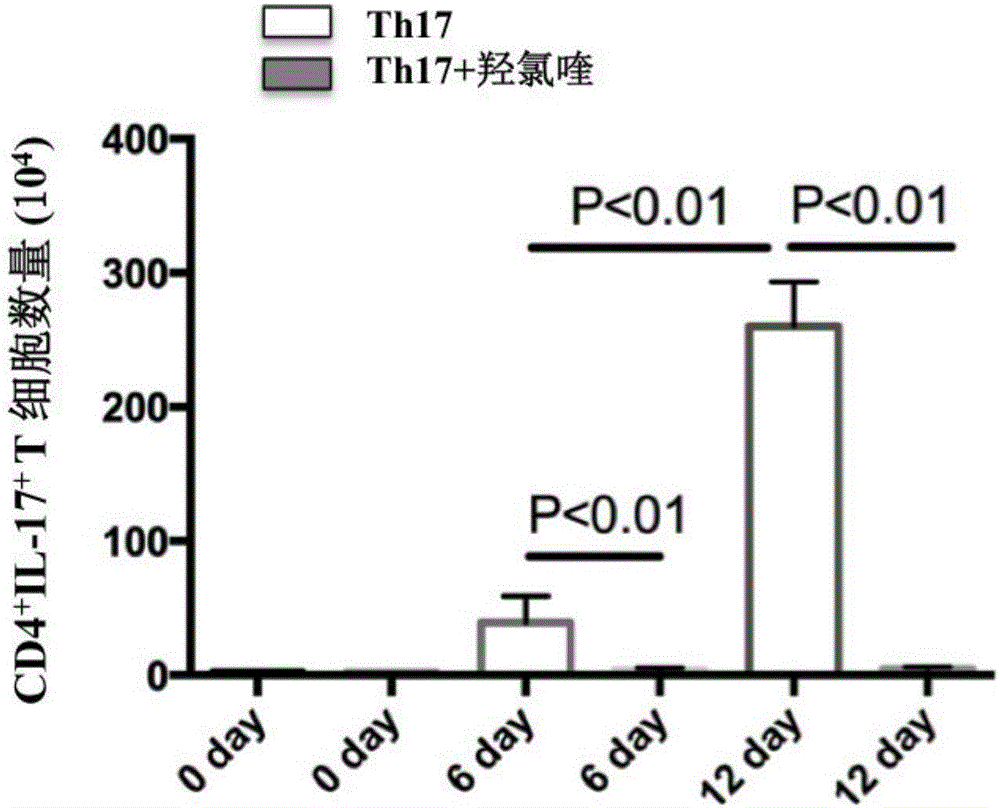
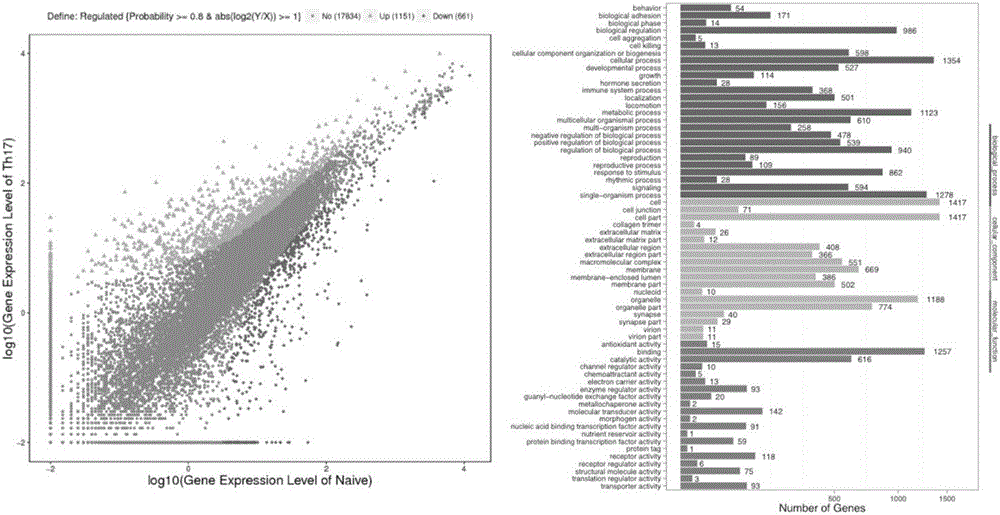
Application of micro RNA group related to Th17 differentiation to preparation of drugs for treatment and effect judgment
InactiveCN105879061AClinical curative effect is convenient and quickConvenient and quick clinical efficacy judgmentOrganic active ingredientsAntipyreticHydroxychloroquineClinical efficacy
The invention provides a micro RNA group related to Th17 differentiation. The micro RNA group comprises micro RNA 4426, micro RNA 3615, micro RNA 106b, micro RNA 590 and micro RNA 573. The micro RNA changes remarkably in the Th17 differentiation process and can be used as Th17 control targets for preparing drugs for treating or preventing Th17-related diseases including lupus erythematosus, dermatomyositis, vasculitis, sicca syndromes, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, scleroderma, multiple sclerosis, malaria, solar dermatitis, eczema, leucoderma, psoriasis, atopic dermatitis, lichen planus, diabetes, coronary heart diseases, hyperlipemia and the like. The micro RNA group is sensitive to hydroxychloroquine intervention and can serve as a sensitive index for judgment of the clinical effect of hydroxychloroquine, and the micro RNA is convenient to test clinically and can be rapidly used for judging the clinical effect of hydroxychloroquine.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
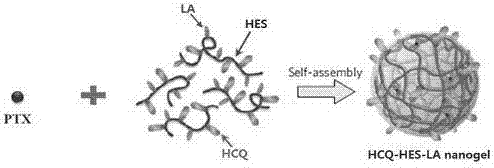
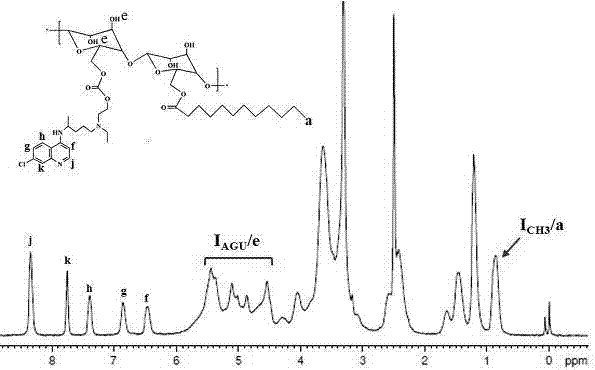
Chloroquine-polymerized nanogel delivery system and preparation method thereof
InactiveCN107375199APrevent proliferationStable structureAerosol deliveryOintment deliveryGene deliveryLymphatic Spread
The invention discloses a chloroquine-polymerized nanogel delivery system and a preparation method thereof. The delivery system contains chloroquine-polymerized nanogel particles, and antitumor medicines which are wrapped with the particles, wherein the chloroquine-polymerized nanogel is prepared through hydroxychloroquine and polysaccharide framework which is modified through a hydrophobic side chain. According to the medicine delivery system, malignant tumor and tumor metastasis are treated synchronously; the signal cascade of cancer cells metastasizing to surrounding and distant sides is stopped while cancer cell proliferation is inhibited, and malignant cancer can be effectively treated.
Owner:CHINA PHARM UNIV
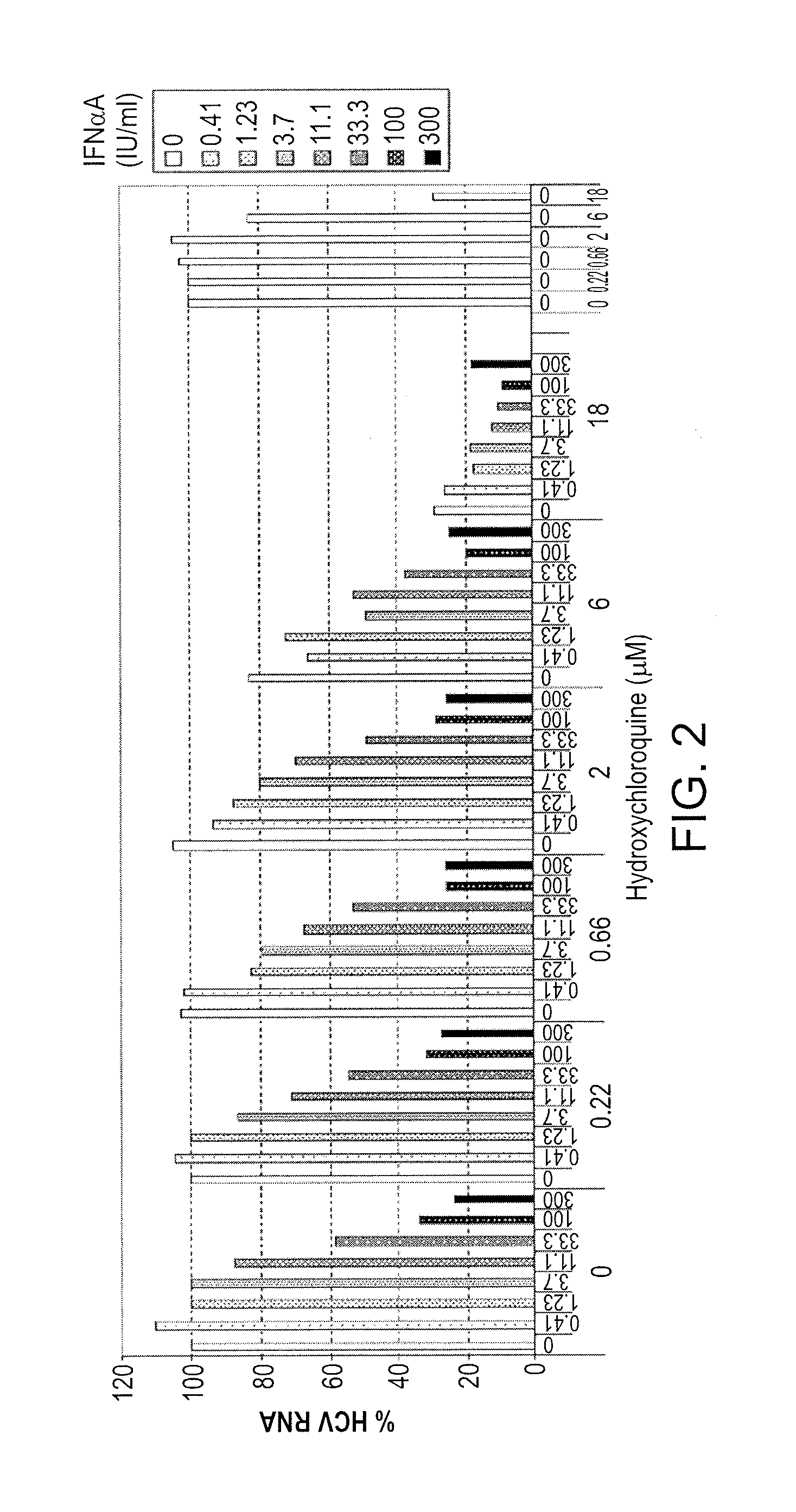
Treatment of hepatitis C virus related diseases using hydroxychloroquine or a combination of hydroxychloroquine and an anti-viral agent
Methods of treating a hepatitis C virus (HCV) related disease, such as HCV infections in subjects non-responsive to anti-HCV therapy, are described herein, comprising administering to the subject a therapeutically effective amount of hydroxychloroquine. An antiviral agent may be co-administered with the hydroxychloroquine. Methods utilizing synergistic combinations of hydroxychloroquine and an antiviral agent are disclosed. Further disclosed are compositions comprising hydroxychloroquine and an antiviral agent, as well as hydroxychloroquine and uses thereof for the treatment of a hepatitis C virus (HCV) related disease.
Owner:PANMED +1
Processes for taste-masking of inhaled formulations
InactiveUS20080138397A1Minimizing bitter tasteMinimizing cough creationBiocideDispersion deliveryPulmonary inhalationThroat irritation
The present invention provides novel processes and methodologies to minimize the bitter or otherwise unpleasant taste, to minimize the tendency to stimulate the cough reflex, or to minimize oropharyngeal deposition of medically-active compounds administered by the pulmonary / inhalation route and to deliver hydroxychloroquine (HCQ) either singularly or in combination with an antimalarial and aminoquinolone by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the bitter or otherwise unpleasant taste of HCQ or any potential to stimulate the cough reflex, and to deliver a dopaminergic compound or its prodrug, including ABT-431 by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate the cough reflex, and to deliver a lantibiotic, including duramycin by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate throat irritation.
Owner:ARADIGM
Treatment of diseases associated with inflammation
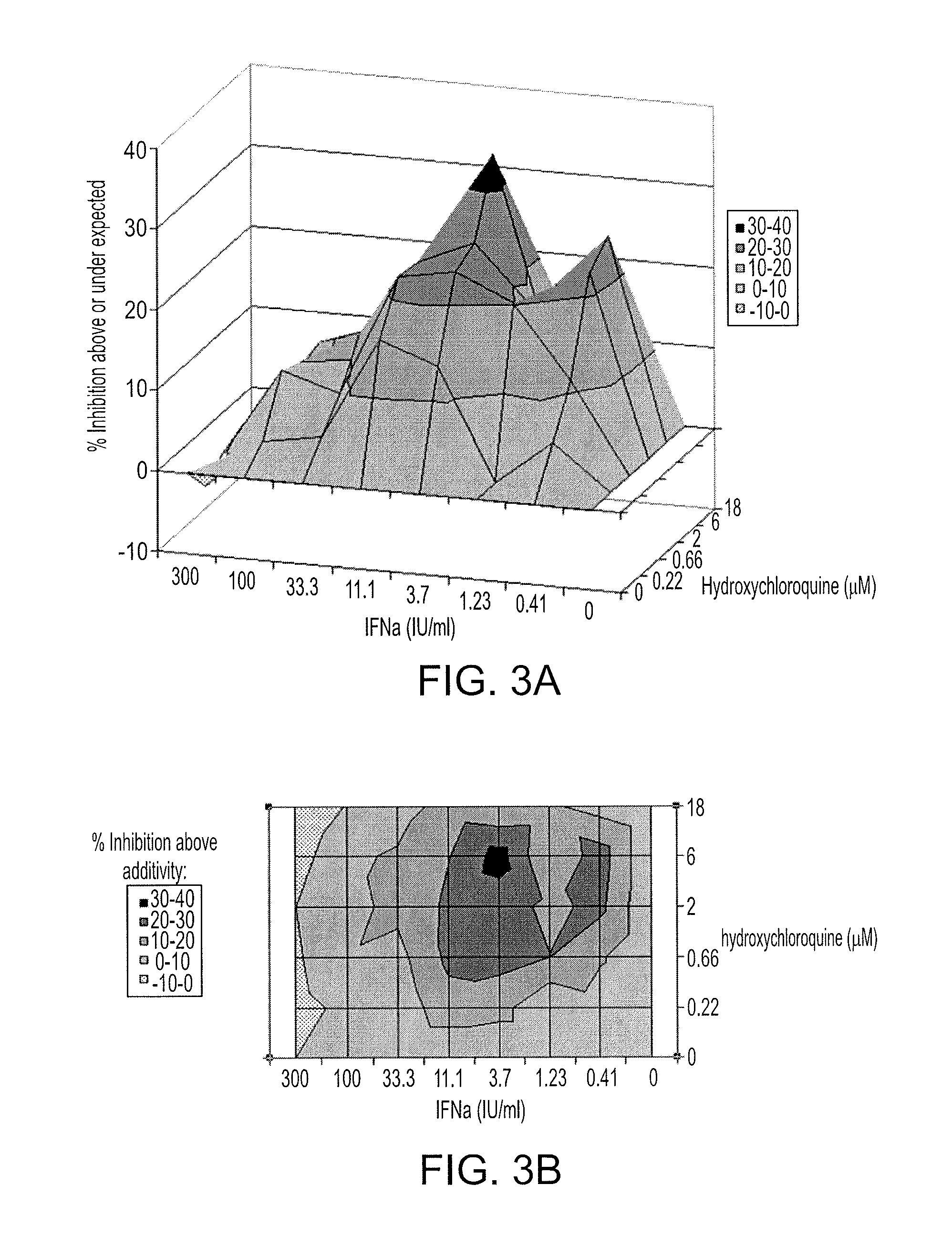
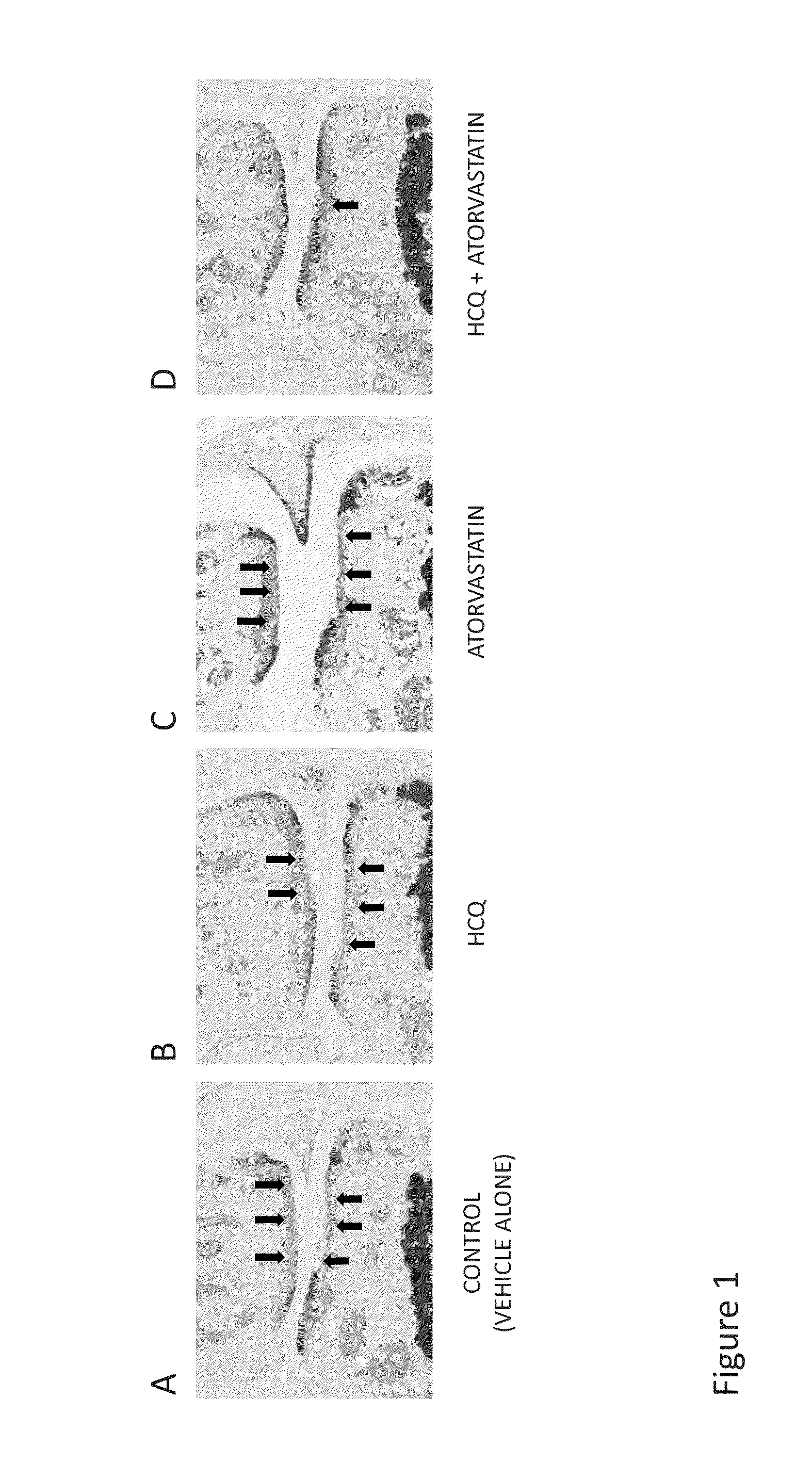
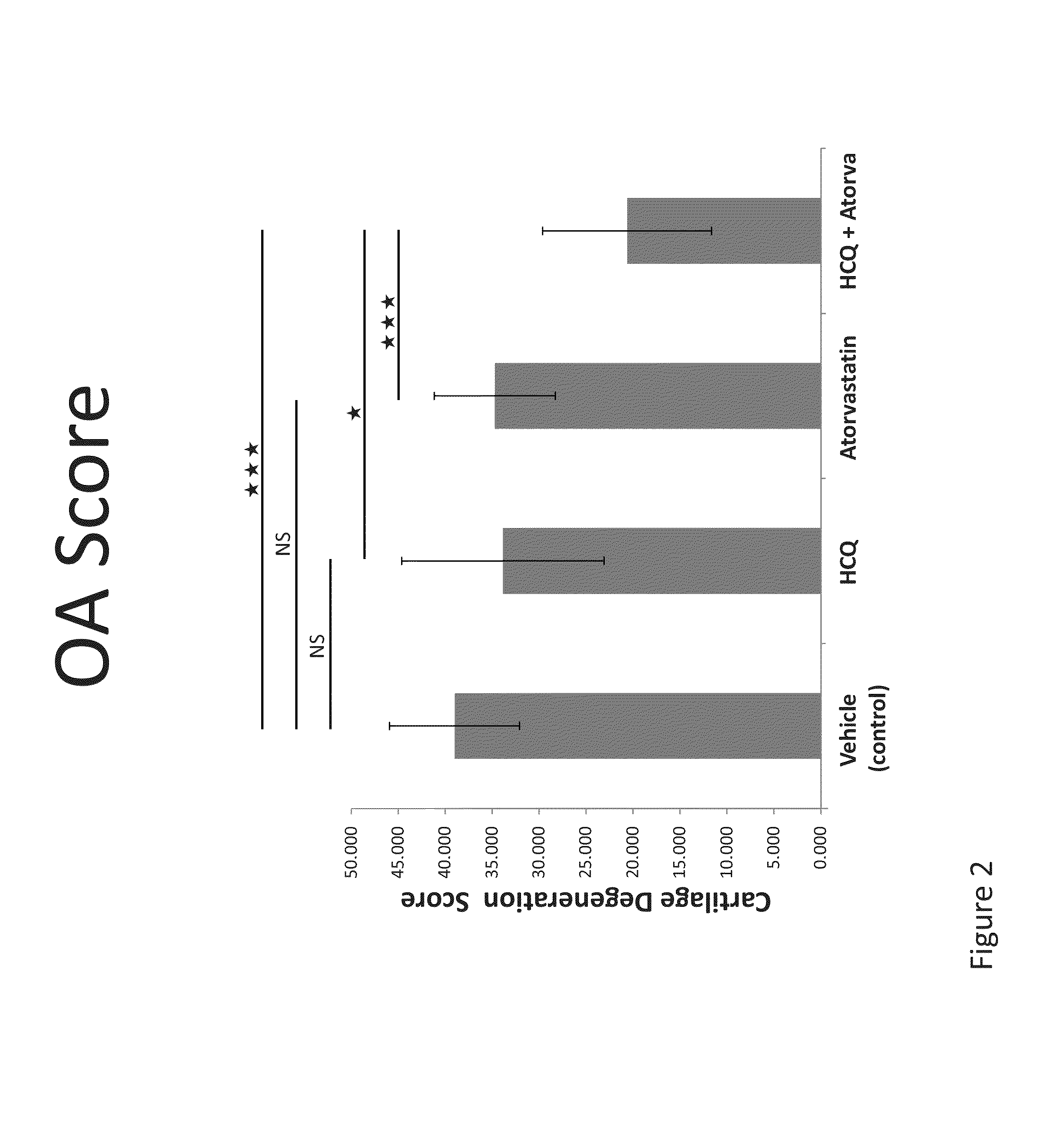
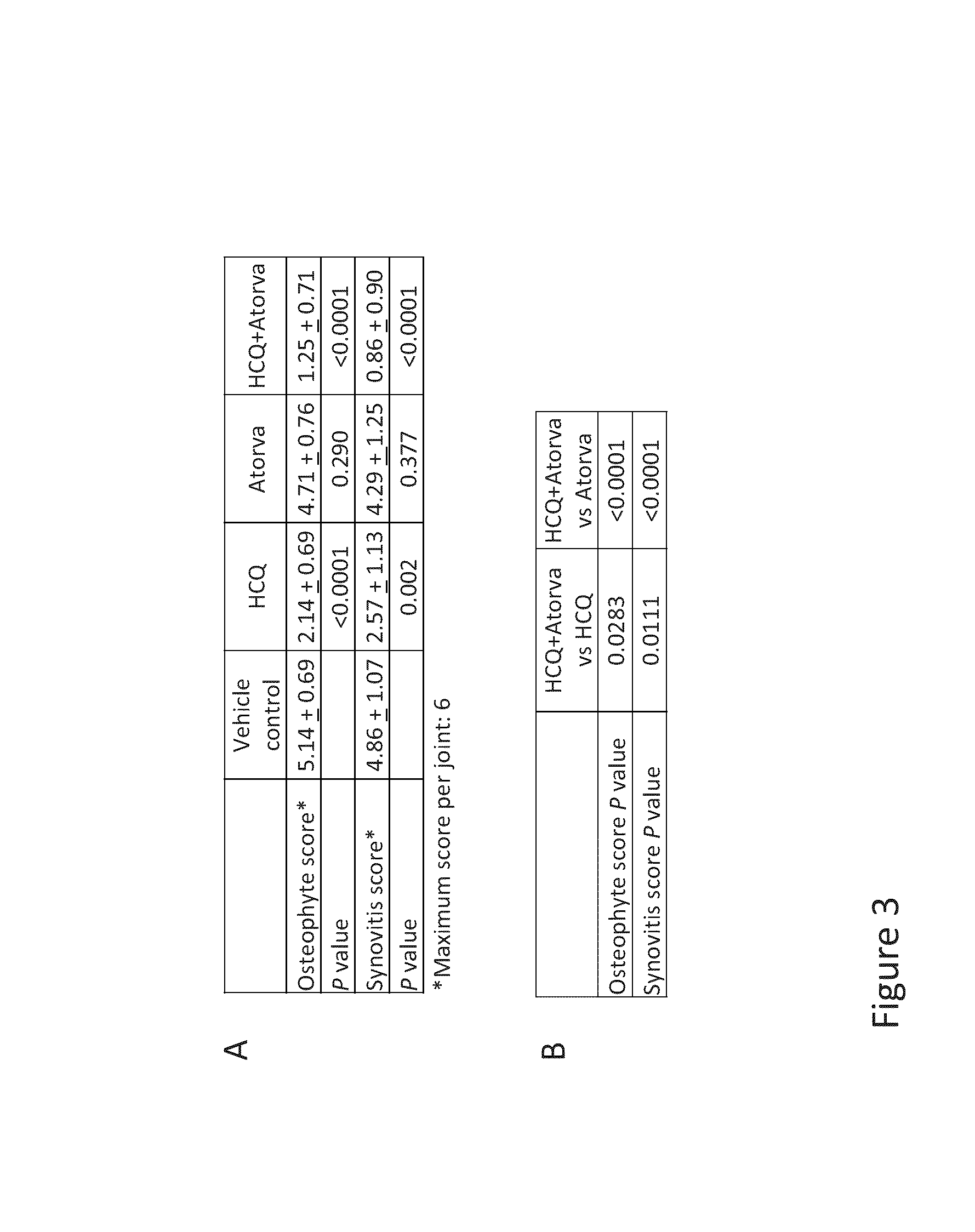
Compositions and methods are provided for preventing or treating the pre-clinical early-stages of inflammatory diseases, including autoimmune diseases, degenerative inflammatory diseases, metabolic inflammatory diseases, chronic infection associated with inflammation, cancer associated with inflammation, and other inflammatory diseases by administration to an individual of an effective dose of a synergistic combination of active agents comprising or consisting essentially of an aminoquinoline, e.g. hydroxychloroquine, and a statin, e.g. atorvastatin. Each or both of the active agents can be formulated in various ways, including without limitation a solid oral dosage form.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Industrial preparation method of hydroxychloroquine sulfate
The invention relates to an industrial preparation method of hydroxychloroquine sulfate, which comprises heating 4, 7-dichloroquinoline and hydroxychloroquine side chain at refluxing temperature to 120-125 DEG C, allowing reaction to obtain hydroxychloroquine, and reacting with sulfuric acid to obtain hydroxychloroquine sulfate. The method can obtain high-purity hydroxychloroquine sulfate with single impurity less than or equal to 0.1% and purity higher than or equal to 99.5%; and has less preparation procedures, simple process, high product yield, good quality, low environmental pollution, no use of highly toxic solvent, and is easy for industrial production.
Owner:CHONGQING KANGLE PHARMA
Compositions and methods for treating and inhibiting viral infections
InactiveUS20140011839A1Inhibition of replicationBiocideOrganic chemistryInfected cellHydroxychloroquine
Compositions and methods for the treatment, as well as the inhibition and prevention, of an infection of the papillomavirus and the epithelial lesions, namely, the warts of the skin and mucosal surfaces, associated therewith, in a mammalian host, as well as methods of inhibiting the replication of a papillomavirus in an infected cell, are provided. The compositions comprise a therapeutically effective amount of an active ingredient comprising at least one compound selected from the group consisting of chloroquine, hydroxychloroquine, amodiaquine, or in each case, a pharmaceutically acceptable salt thereof. The methods comprise topically administering a therapeutically and / or antivirally effective amount of such a compound to a mammalian host, such as a human being, in need of such treatment, although alternatively other routes of administration may be used, including but not limited to transdermal, transmucosal, respiratory, and by injection. The compositions optionally also comprise one or more pharmaceutically acceptable non-active ingredients.
Owner:GRACELAND BIOGENESIS INT CATALYST LLC
Compositions and methods for treating warts associated with viral infections
The invention provides compositions and methods for the treatment, as well as the inhibition and prevention, of an infection of the human papillomavirus and the epithelial lesions, namely, the warts of the skin and mucosal surfaces, associated therewith. The compositions comprise a therapeutically effective amount of an active ingredient comprising a compound selected from the group consisting of chloroquine, hydroxychloroquine, chloroquine combined with amodiaquine, and hydroxychloroquine combined with amodiaquine, or in each case, a pharmaceutically acceptable salt thereof. The methods comprise topically administering a therapeutically and / or antivirally effective amount of such a compound to a human being in need of such treatment, although in certain of the compounds may alternatively be administered subcutaneously or transdermally. The compositions may optionally also comprise one or more pharmaceutically acceptable non-active ingredients.
Owner:OBI JUSTICE E
Compositions and methods for treating warts associated with viral infections
The invention provides compositions and methods for the treatment, as well as the inhibition and prevention, of an infection of the human papillomavirus and the epithelial lesions, namely, the warts of the skin and mucosal surfaces, associated therewith. The compositions comprise a therapeutically effective amount of an active ingredient comprising at least one compound selected from the group consisting of chloroquine, hydroxychloroquine, amodiaquine, or in each case, a pharmaceutically acceptable salt thereof. The methods comprise topically administering a therapeutically and / or antivirally effective amount of such a compound to a human being in need of such treatment, although in certain of the compounds may alternatively be administered subcutaneously or transdermally. The compositions may optionally also comprise one or more pharmaceutically acceptable non-active ingredients.
Owner:OBI JUSTICE E
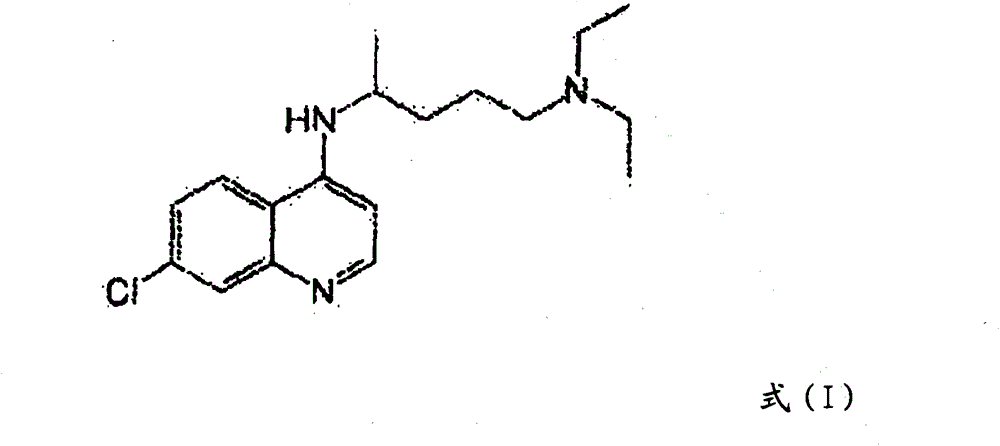
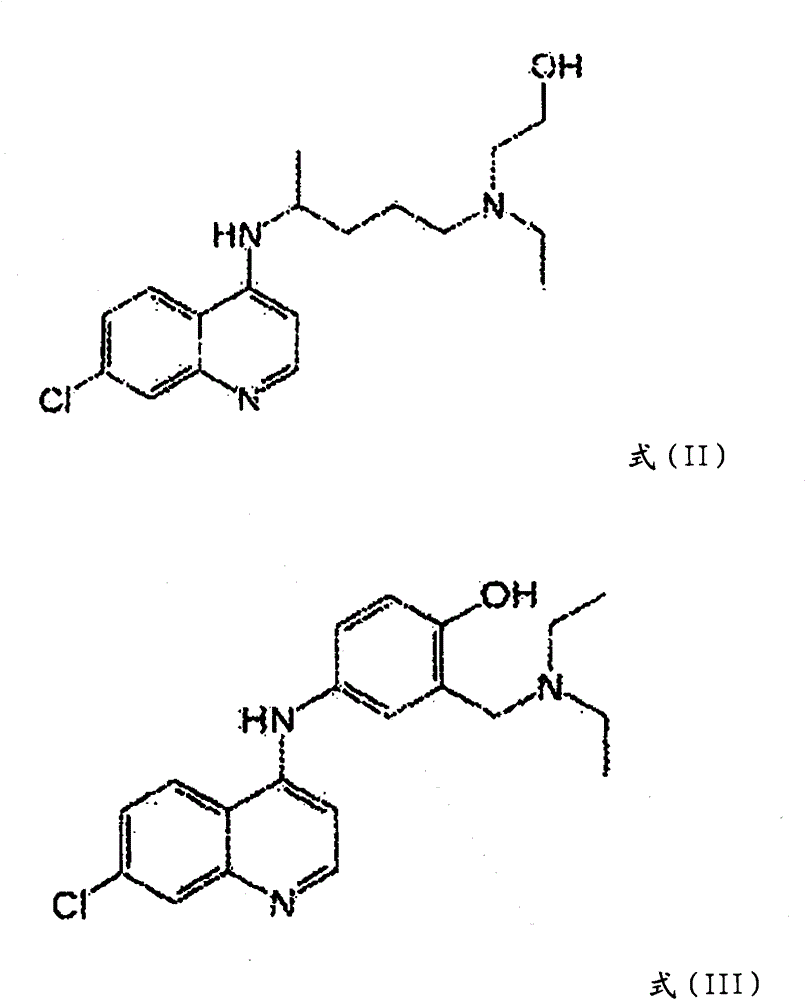
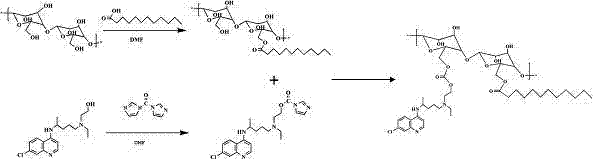
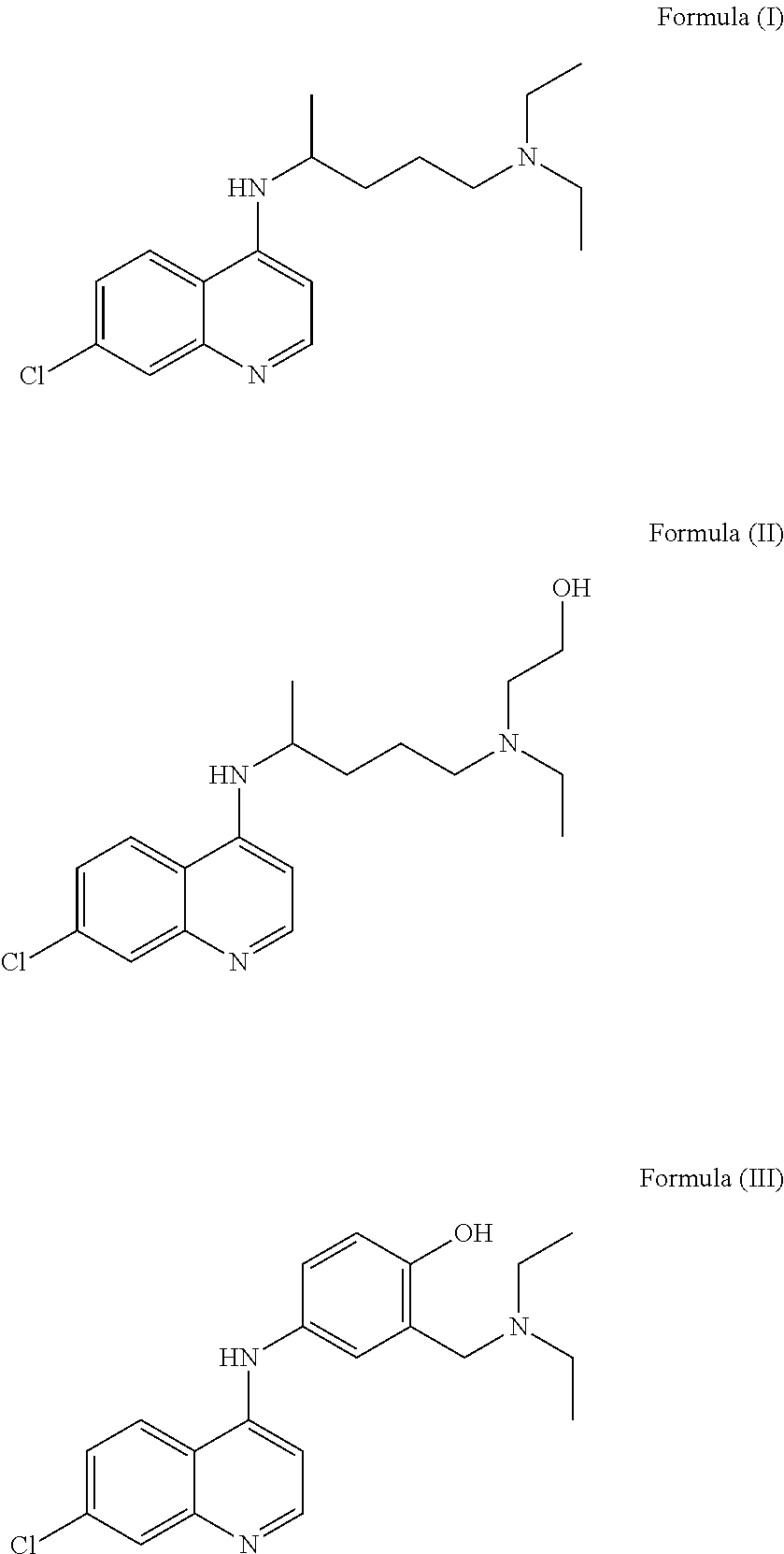
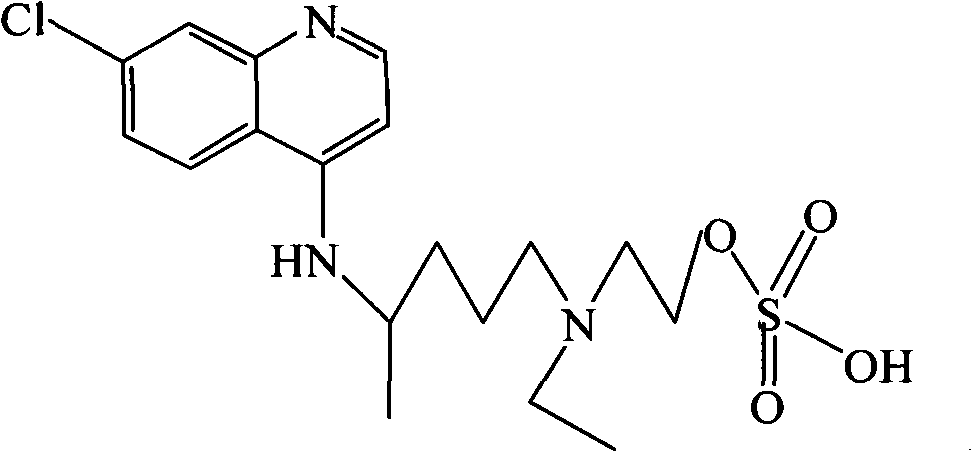
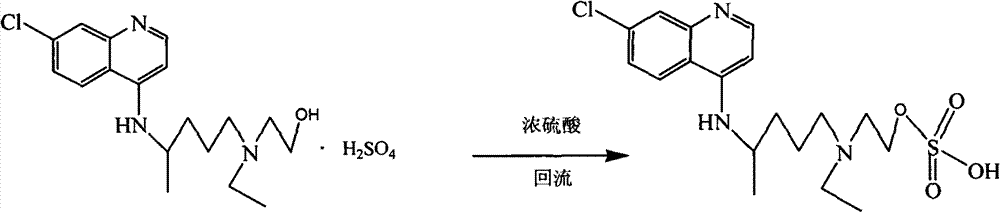
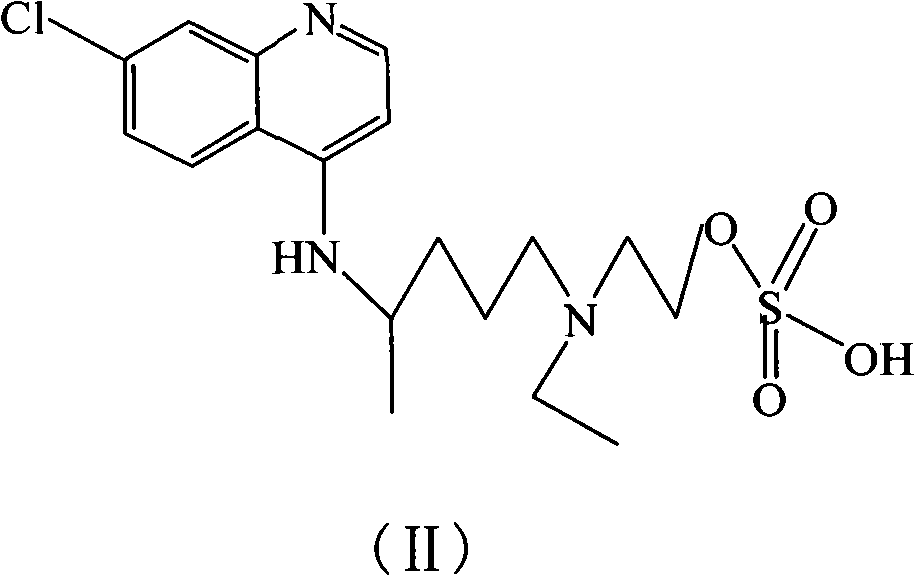
Hydroxychloroquine derivative and its synthetic method
The invention discloses a hydroxychloroquine derivative II, which is 2-[[4-[7-chloro-4-quinolyl)amino]pentyl]ethylamino]-ethyl sulfate, and its synthetic method; the hydroxychloroquine derivative II is a sulphoacid esterification product of hydroxychloroquine, which is one of main impurities of the hydroxychloroquine medicine and its preparation; the compound II can be used for analyzing and detecting the purity of hydroxychloroquine and its salt, and can be used for controlling the quality of the hydroxychloroquine and its salt; The invention discloses the synthetic method of the compound II, and an application of the prepared impurities comparison product used in the quality control of hydroxychloroquine and its preparation.
Owner:WUHAN WUYAO SCI & TECH
A kind of industrialized preparation method of hydroxychloroquine sulfate
The invention provides an industrial production method for hydroxychloroquine sulfate, which includes the following steps: enabling 4.7-dichloroquinoxaline and 5-(N-ethyl-N-ethoxyl)-2-amino pentane to react under gas shield for 13-24 h at a gradually increased temperature of 120-130 DEG C to obtain hydroxychloroquine; preparing the hydroxychloroquine sulfate after the reaction between the hydroxychloroquine and an alcohol sulfate solution at the temperature of 20-30 DEG C. According to the method, the yield of the obtained crude product of the hydroxychloroquine is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate HPLC is not smaller than 99.5%, the yield of single impurity is not larger than 0.1%, so that requirements of United States Pharmacopeia is met; the novel method is simple in procedure, is environment-friendly and easy in industrial production.
Owner:WUHAN WUYAO PHARMA
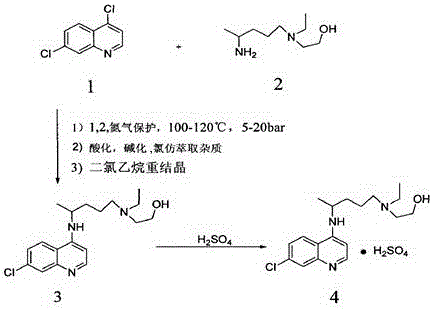
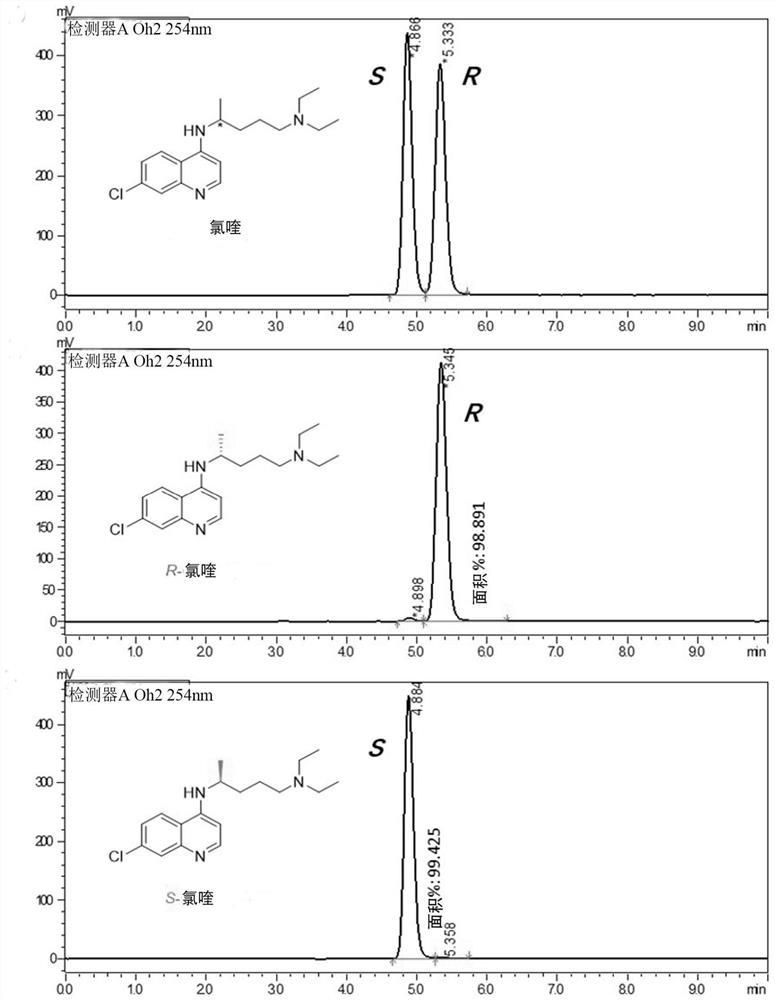
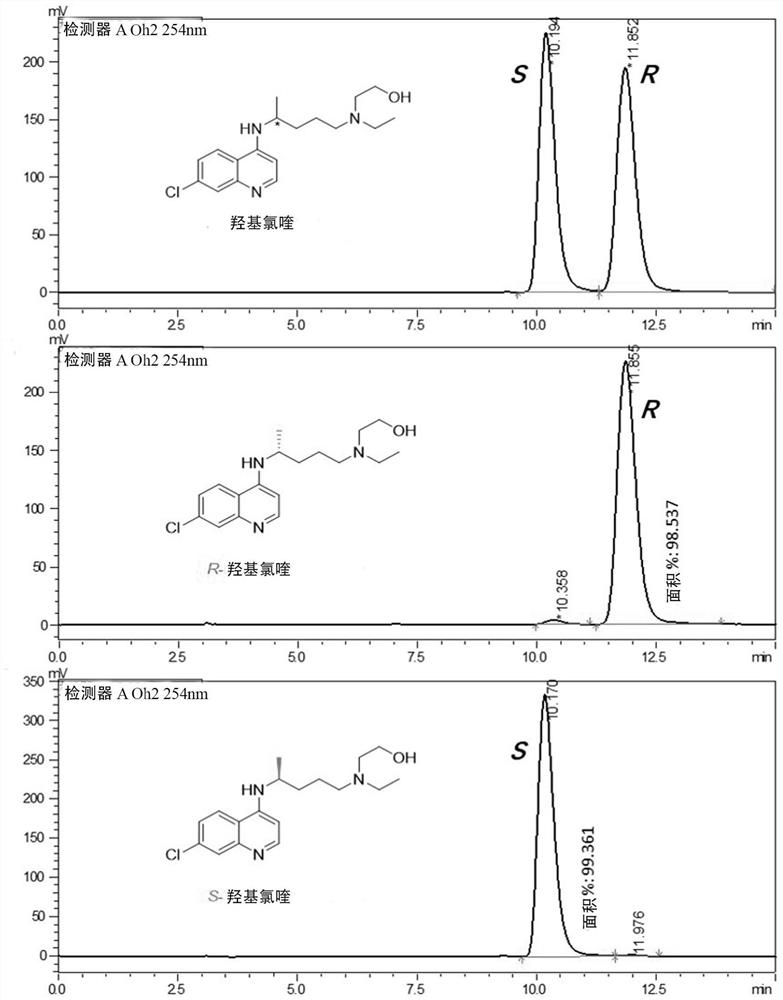
Optically active chloroquine and hydroxychloroquine and analogues thereof, and preparation method, composition and application of optically active chloroquine and hydroxychloroquine
PendingCN112457244AHigh optical puritySimple and fast operationOrganic active ingredientsOrganic chemistryAutoimmune conditionHydroxychloroquine
The invention provides a rapid and simple method for preparing optically active chloroquine, hydroxychloroquine and analogues thereof, which comprises the following steps: reacting racemates of chloroquine, hydroxychloroquine and analogues thereof with an acidic chiral resolution reagent to generate corresponding salts, and separating and purifying to obtain optically pure salts of chloroquine, hydroxychloroquine and analogues thereof, and reacting with alkali to obtain (R)- or (S)- chloroquine with high optical purity and (R)- or (S)- hydroxychloroquine and analogues thereof. The method is simple and convenient to operate and low in cost, the enantiomer purity can reach 99.9% ee, and industrial production of chloroquine, hydroxychloroquine and analogues thereof with single chiral configuration is easy to realize. The invention also provides (R)- or (S)- chloroquine and (R)- or (S)- hydroxychloroquine and analogues thereof, pharmaceutical compositions and uses thereof, optically activechloroquine and hydroxychloroquine and analogues thereof reduce toxic and side effects, and have better treatment effects on coronavirus, influenza virus and autoimmune diseases.
Owner:瀚海新拓(杭州)生物医药有限公司
Uses for anti-malarial therapeutic agents
A diversity of inflammatory diseases can be treated via local delivery to the patient of a composition containing a therapeutically effective amount of an anti-malarial agent. In a preferred embodiment of the method of the invention, a pulmonary inflammatory condition, such as asthma, is treated by inhalation of an aerosolized anti-malarial agent, such as hydroxychloroquine.
Owner:CHAROUS LAUREN
A kind of preparation method of hydroxychloroquine sulfate
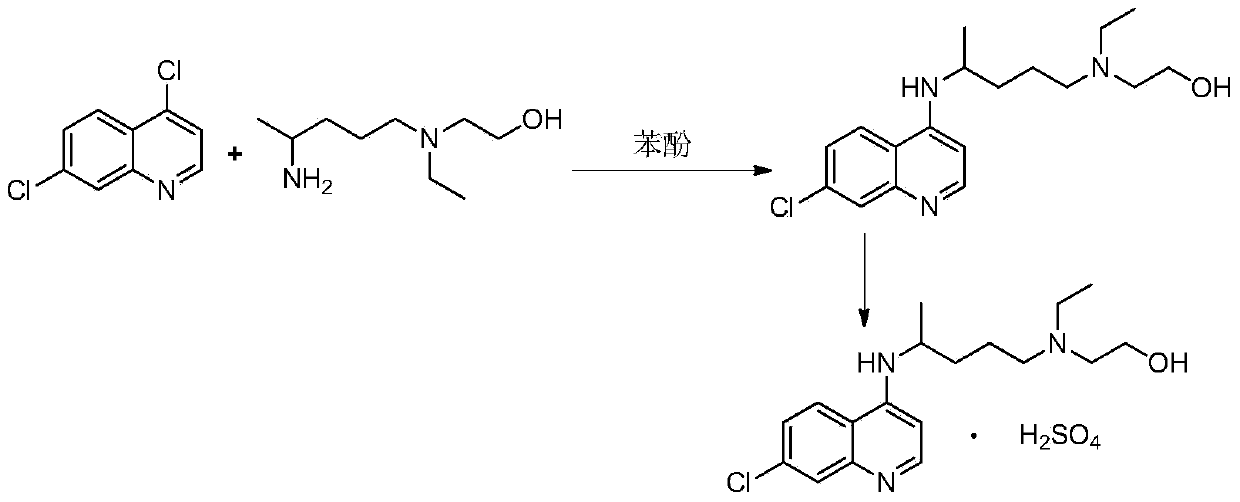
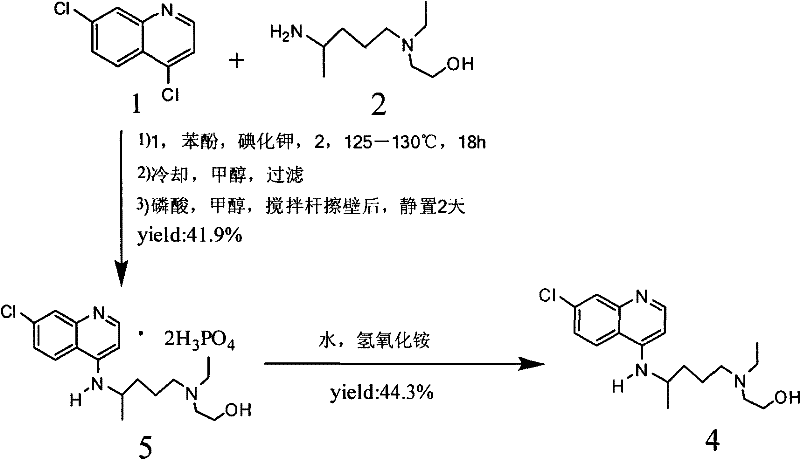
The invention discloses a preparation method of hydroxychloroquine sulfate. The preparation method is characterized by comprising the following steps: condensing 4,7-dichloroquinoline serving as an initial raw material and a hydroxychloroquine side chain under the action of a catalyst, so as to obtain hydroxychloroquine; and reacting hydroxychloroquine with sulfuric acid, so as to prepare the hydroxychloroquine sulfate. According to the method disclosed by the invention, the defects in the prior art are overcome. The method has the advantages that the yield of the prepared hydroxychloroquine sulfate crude product is greater than or equal to 85%, the yield of hydroxychloroquine sulfate is greater than or equal to 94%; the total yield is greater than or equal to 80%; the purity of the prepared hydroxychloroquine sulfate HPLC is greater than or equal to 99.6%; the maximum single impurity is smaller than 0.1%; the method accords with the requirements of United States pharmacopeia, is short in reaction step, and simple in the whole technological operation; and the obtained product is high in quality, high in yield, and relatively suitable for industrial production.
Owner:TSINGHUA TONGFANG CO LTD
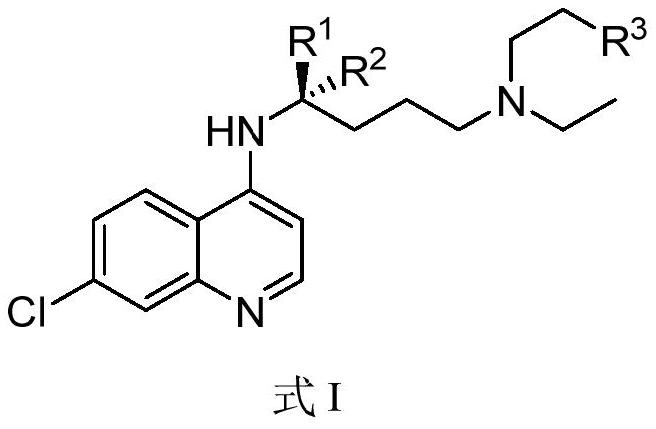
Chiral chloroquine, hydroxychloroquine and derivatives thereof as well as preparation method and application of chiral chloroquine, hydroxychloroquine and derivatives thereof
ActiveCN111620815AEasy to operateSuppress deathOrganic active ingredientsOrganic chemistry methodsHydroxychloroquineFluid phase
The invention belongs to the field of drug synthesis, and discloses a compound with a structure as shown in a formula I (See the specification) and pharmaceutically acceptable salt, tautomer, polymorphic substance, isomer and solvate thereof. Secondly, the invention discloses a method for preparing chiral chloroquine and hydroxychloroquine through chiral high performance liquid chromatography. Finally, the invention discloses application of chiral chloroquine, hydroxychloroquine and salt derivatives thereof in preparation of drugs for treating novel coronavirus pneumonia.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
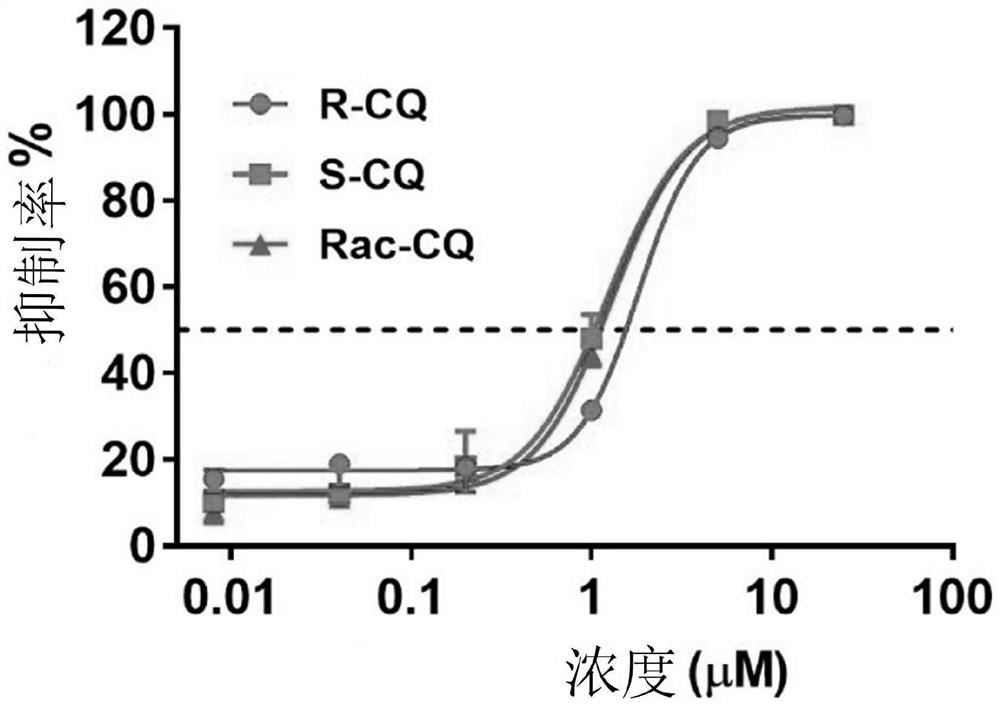
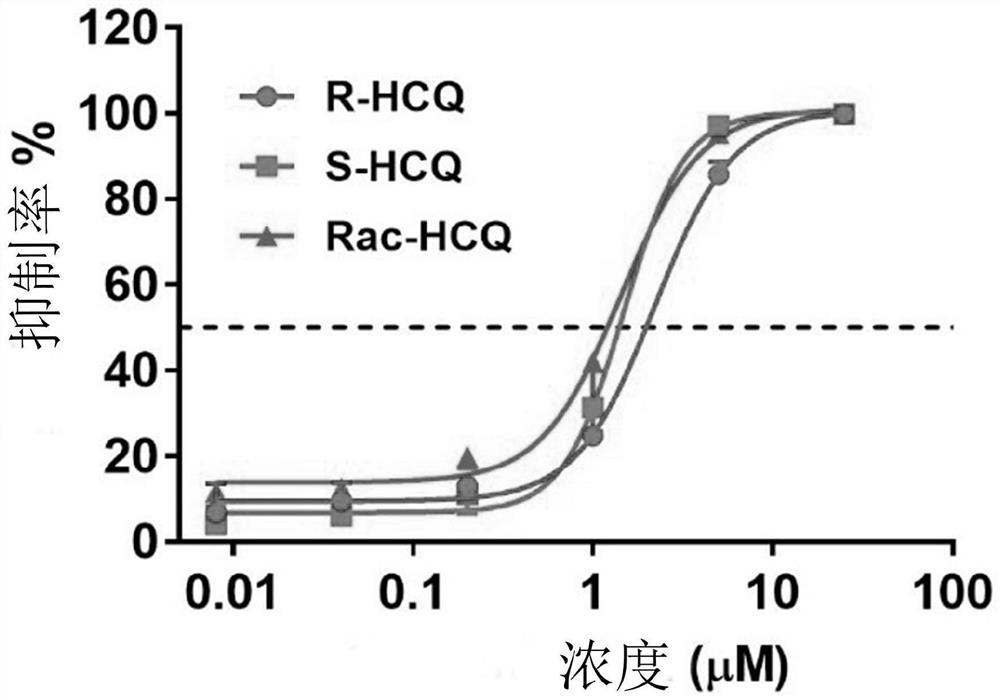
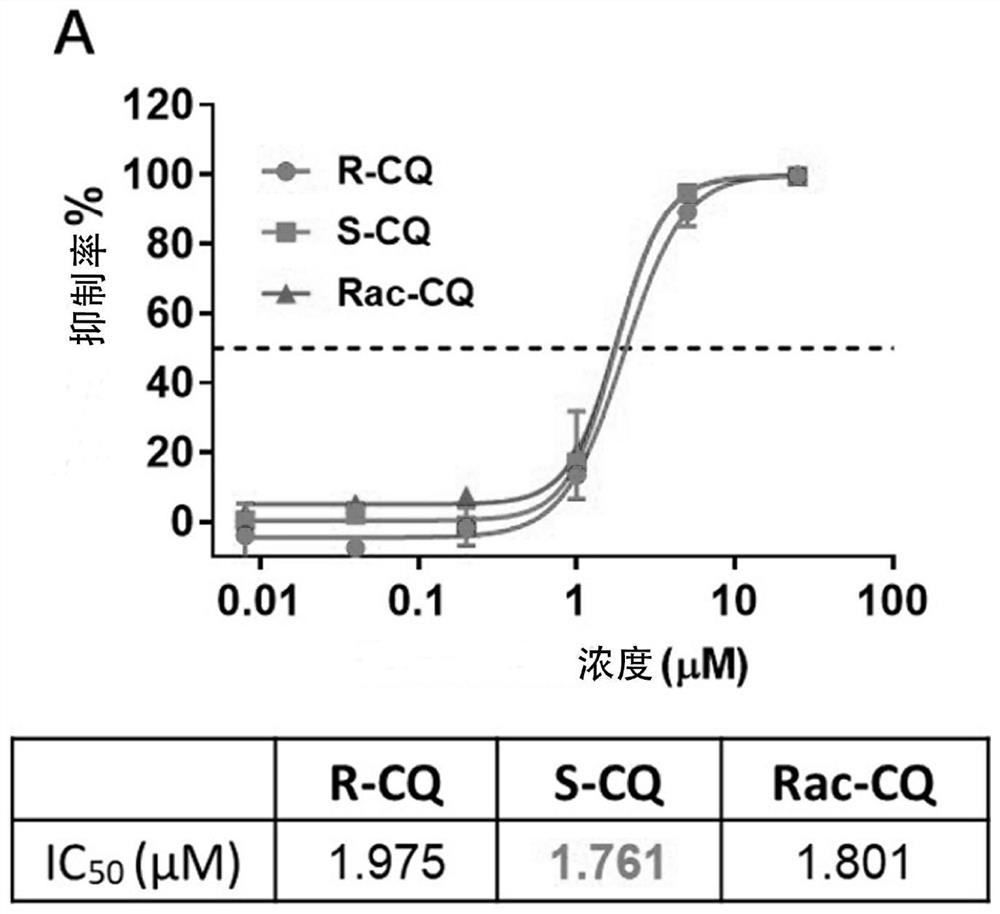
Application of chiral chloroquine, hydroxychloroquine or salt of the chiral chloroquine and hydroxychloroquine as anti-coronavirus drug target 3CL hydrolase inhibitor for reducing cardiotoxicity
ActiveCN111803501AEnhanced inhibitory effectSmall inhibition rateOrganic active ingredientsInorganic active ingredientsHydroxychloroquineHydrolase inhibitor
The invention discloses an application of chiral chloroquine, hydroxychloroquine or pharmaceutically acceptable salts of the chiral chloroquine and hydroxychloroquine in preparation of drugs used forpreventing and / or treating coronavirus pneumonia by using a coronavirus key drug target 3CL hydrolase (Mpro) as an action target. The chiral chloroquine and hydroxychloroquine have high bonding strength with the Mpro causing inflammation of the lung and the like; the activity of the Mpro can be significantly inhibited; and the chiral chloroquine and hydroxychloroquine are indicated to have the effect of preventing and treating pneumonia caused by coronaviruses and be able to be used as anti-pneumonia drugs. Through evaluation on the inhibitory activity of an hERG potassium ion channel, the concentration at which the chloroquine, hydroxychloroquine and enantiomers of the chloroquine and hydroxychloroquine are likely to generate cardiotoxicity to the hERG potassium ion channel is provided. The chiral chloroquine and hydroxychloroquine are prepared through chiral high-performance liquid chromatography and chiral synthesis; S-configuration chloroquine, hydroxychloroquine or salts of the chloroquine and hydroxychloroquine can be selected as a drug independently, or form a pharmaceutical composition for treating diseases caused by the coronaviruses; and due to higher activity and low cardiotoxicity of the chloroquine, hydroxychloroquine or salts of the chloroquine and hydroxychloroquine, the administration dosage range is greatly widened.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA +1
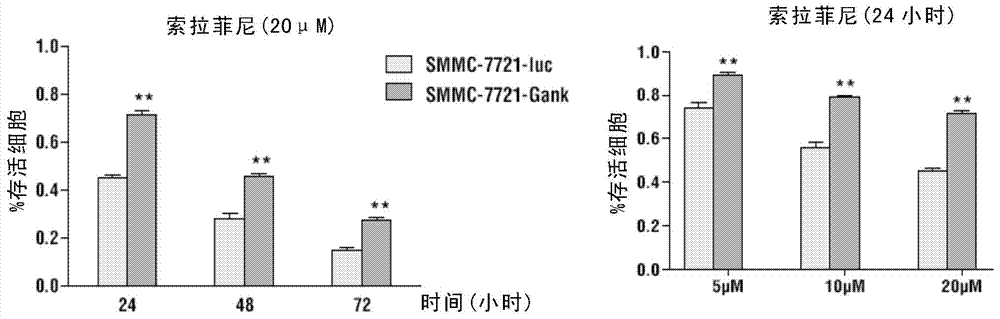
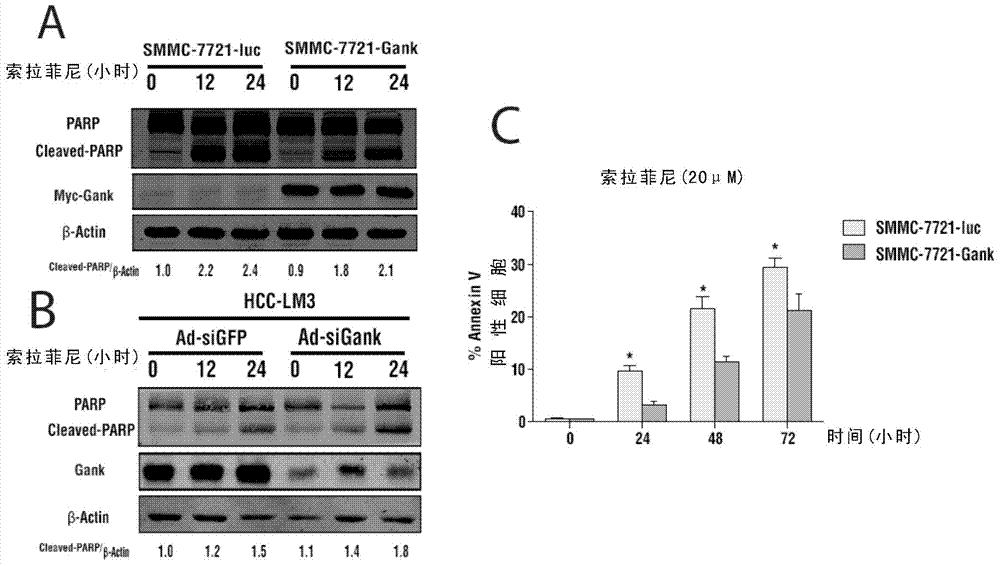
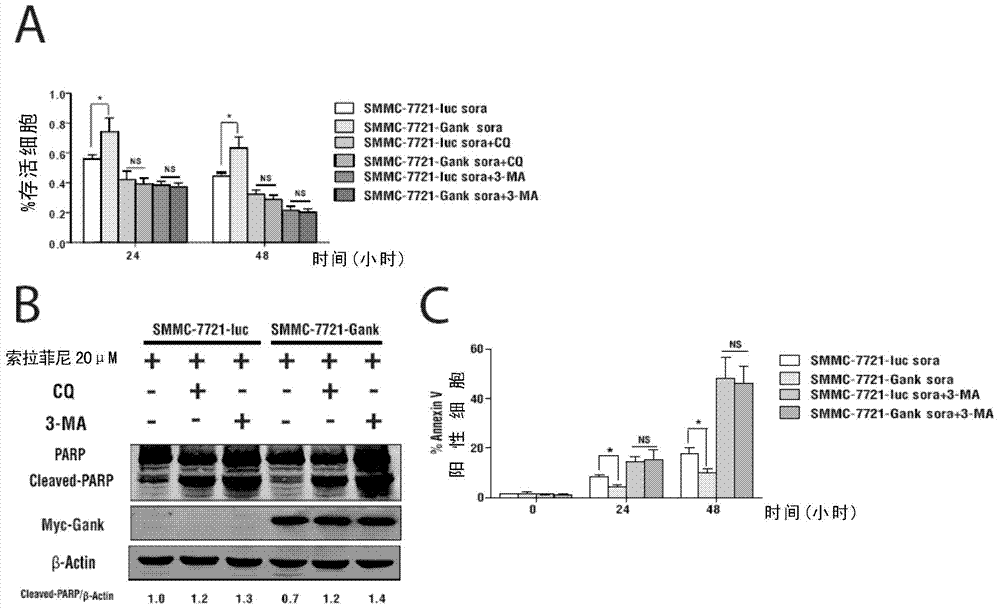
Classification diagnostic kit for liver cancer Sorafenib personalized treatment
The invention provides a classification diagnostic kit for liver cancer Sorafenib personalized treatment. Gankyrin overexpression can obviously restrain cell death and subcutaneous tumor proliferation, caused by Sorafenib, but if hydroxychloroquine is combined, drug resistant reaction caused by Gankyrin overexpression is obviously restrained. The classification diagnostic kit can provide new reference for Sorafenib clinical application.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Processes for taste-masking of inhaled formulations
InactiveUS20110104259A1Reduce interactionReduce concentrationDispersion deliveryAerosol deliveryThroat irritationHydroxychloroquine
The present invention provides novel processes and methodologies to minimize the bitter or otherwise unpleasant taste, to minimize the tendency to stimulate the cough reflex, or to minimize oropharyngeal deposition of medically-active compounds administered by the pulmonary / inhalation route and to deliver hydroxychloroquine (HCQ) either singularly or in combination with an antimalarial and aminoquinolone by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the bitter or otherwise unpleasant taste of HCQ or any potential to stimulate the cough reflex, and to deliver a dopaminergic compound or its prodrug, including ABT-431 by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate the cough reflex, and to deliver a lantibiotic, including duramycin by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate throat irritation.
Owner:ARADIGM
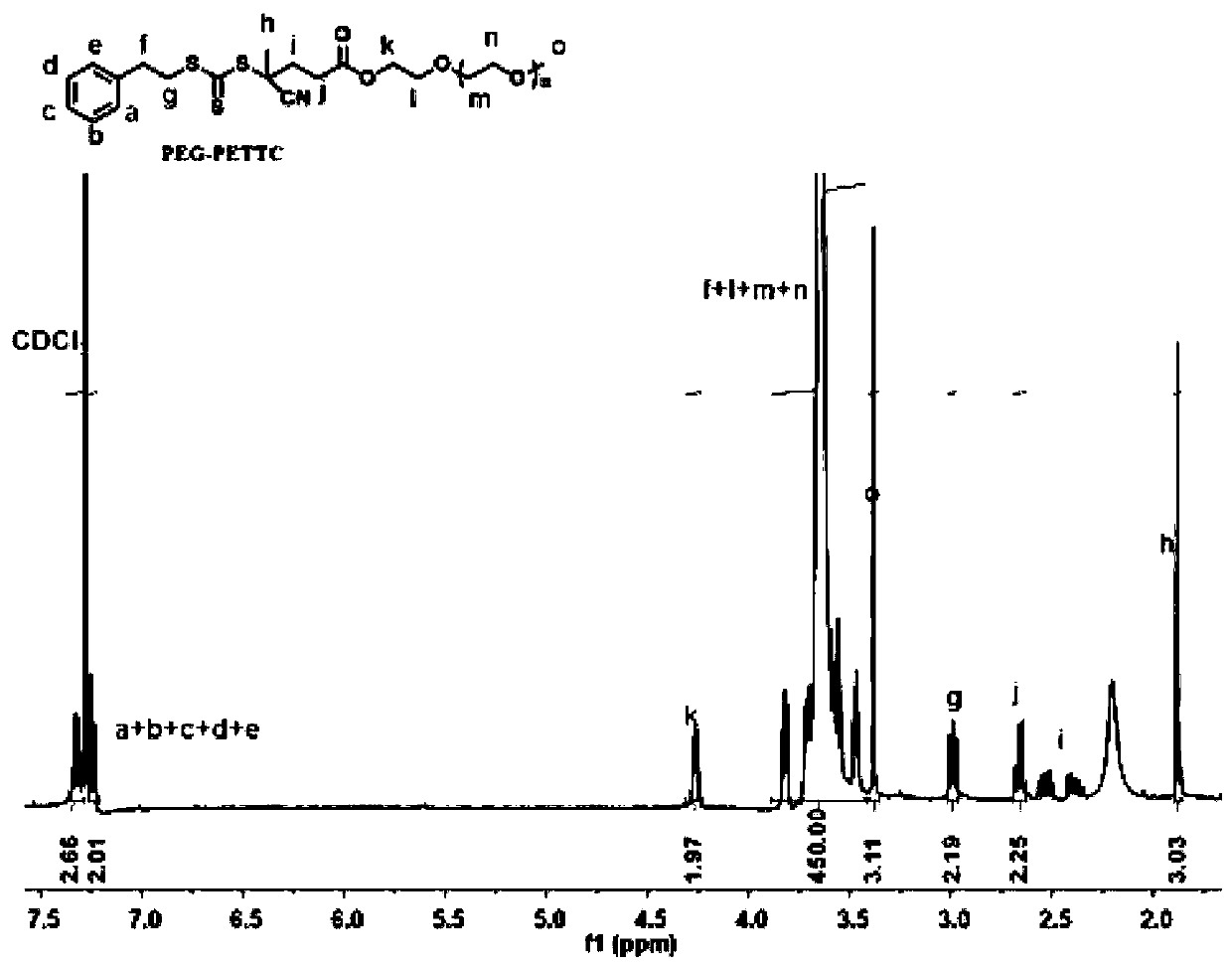
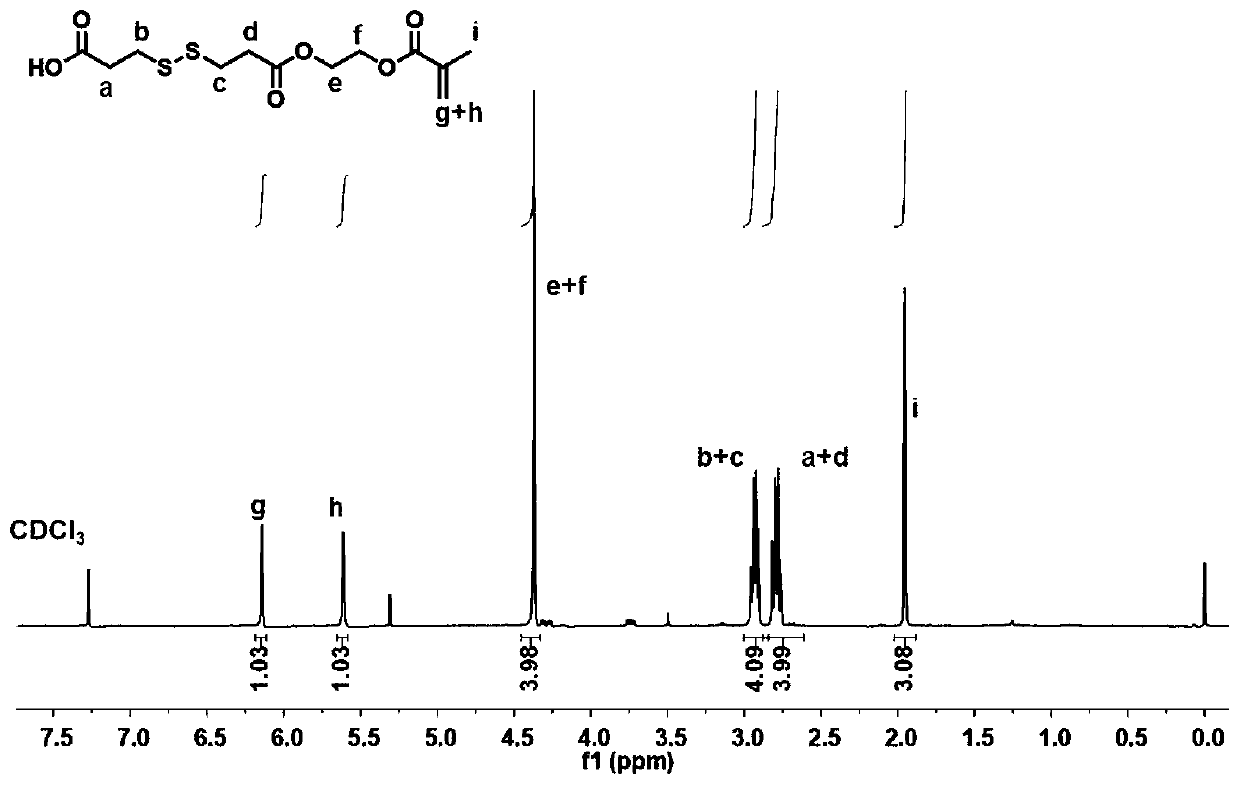
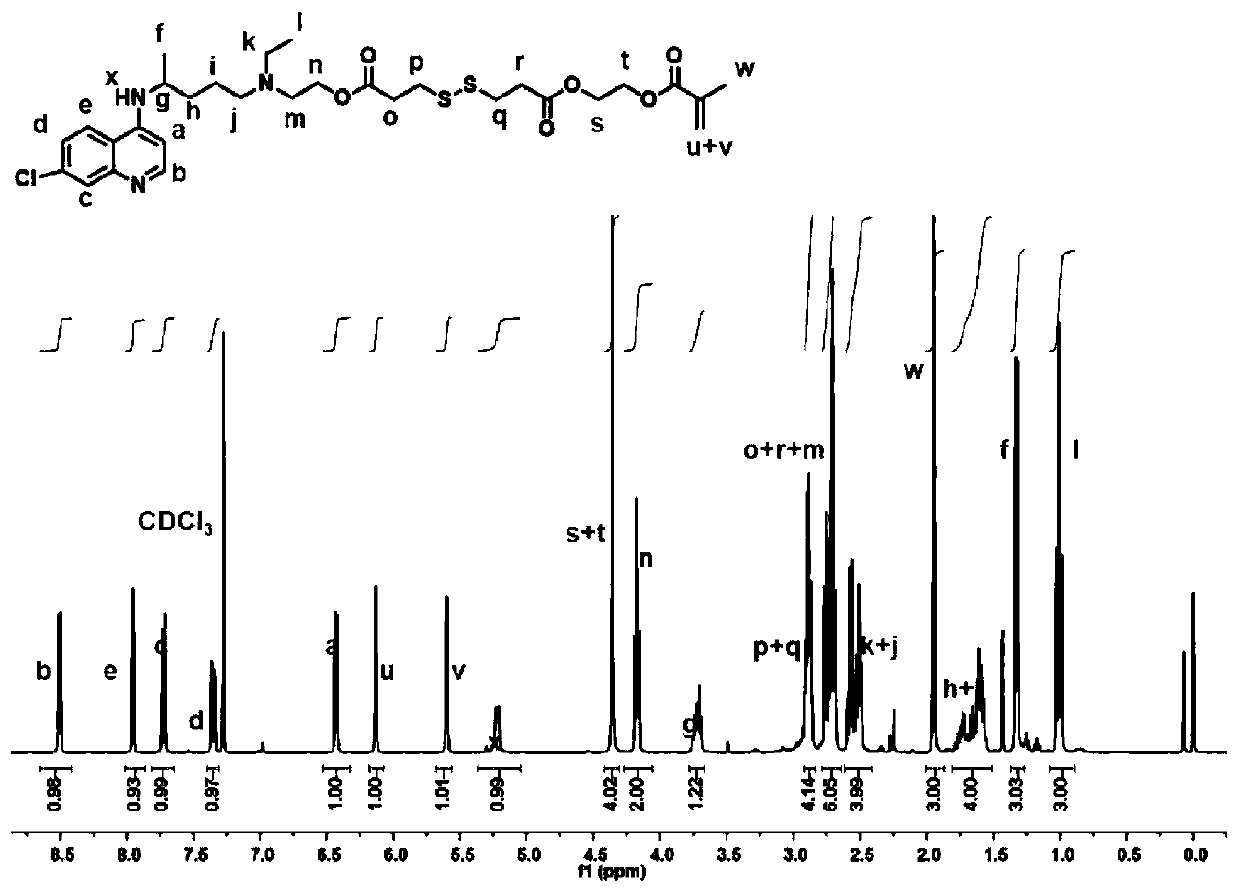
Hydroxychloroquine amphiphilic polymer drug precursor, preparation method and application thereof
ActiveCN110694076AGive full play to the pharmacological effectImprove pharmacokinetic behaviorPowder deliveryOrganic active ingredientsPolymer scienceHydroxychloroquine
The present invention discloses a hydroxychloroquine amphiphilic polymer drug precursor. The hydroxychloroquine amphiphilic polymer drug precursor is composed of hydrophilic polyethylene glycol monomethyl ether and a hydrophobic hydroxychloroquine polymer. The hydroxychloroquine polymer is bonded to the polyethylene glycol monomethyl ether via a cleavable chemical bond. The hydroxychloroquine amphiphilic polymer drug precursor can be self-assembled into nanoparticles with a particle sizeof 20-300 nm in water, which improves pharmacokinetic behaviors of free hydroxychloroquine and enables the hydroxychloroquine amphiphilic polymer drug precursor to have significant long-cycle characteristics. At the same time, the hydroxychloroquine amphiphilic polymer drug precursor nanoparticles can loada variety of hydrophobic drugs, including camptothecin and derivatives thereof, paclitaxel, doxorubicin and bortezomib as well as various types of negatively charged drug molecules, and have a wide range of application values.
Owner:ZHEJIANG UNIV
Compositions and Methods for Treating and Inhibiting Viral Infections
InactiveUS20170296530A1Avoid regrowthAvoid formingInorganic non-active ingredientsSuppositories deliveryInfected cellHydroxychloroquine
Compositions and methods for the treatment, as well as the inhibition and prevention, of an infection of the papillomavirus and the epithelial lesions, namely, the warts of the skin and mucosal surfaces, associated therewith, in a mammalian host, as well as methods of inhibiting the replication of a papillomavirus in an infected cell, are provided. The compositions comprise a therapeutically effective amount of an active ingredient comprising at least one compound selected from the group consisting of chloroquine, hydroxychloroquine, amodiaquine, or in each case, a pharmaceutically acceptable salt thereof. The methods comprise topically administering a therapeutically and / or antivirally effective amount of such a compound to a mammalian host, such as a human being, in need of such treatment, although alternatively other routes of administration may be used, including but not limited to transdermal, transmucosal, respiratory, and by injection. The compositions optionally also comprise one or more pharmaceutically acceptable non-active ingredients.
Owner:OBI JUSTICE E
Application of 4-aminoquinoline compound in treatment of coronavirus infection
The invention relates to hydroxychloroquine or chloroquine, or a geometrical isomer thereof, or a pharmaceutically acceptable salt of the hydroxychloroquine or chloroquine, and / or a solvate of the hydroxychloroquine or chloroquine, and / or a hydrate of the hydroxychloroquine or chloroquine, a pharmaceutical composition containing the compound, and an application of the hydroxychloroquine or chloroquine to treatment of diseases or infection caused by SARS-CoV-2.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com