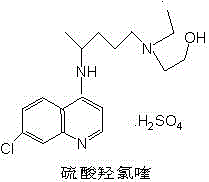
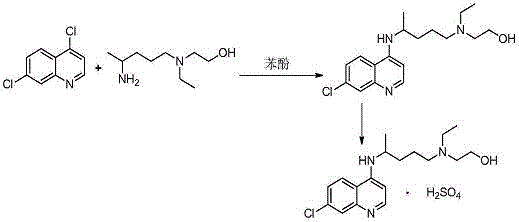
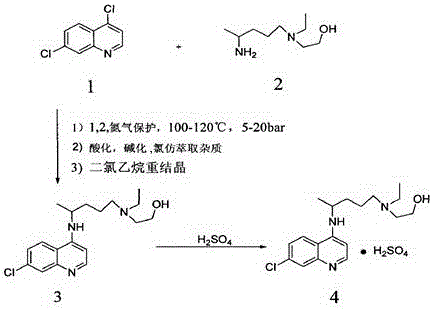
A kind of preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine, which is applied in the fields of medicine and chemical industry, can solve the problems of producing dimethyl sulfate and diethyl sulfate, being unsuitable for industrialized production, and not being able to achieve them, so as to reduce the number of washings and avoid toxic Substance risk, easy operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 hydroxychloroquine sulfate
[0034] 1.1 The preparation of hydroxychloroquine
[0035]In a three-neck round bottom flask, add 4,7-dichloroquinoline (198.0g, 1.0mol), hydroxychloroquine side chain (182.7g, 1.05mol) and isopropyl acetate 1089g, slowly add sodium ethoxide (13.6g, 0.2mol), slowly heat up to reflux under stirring conditions, then distill out isopropyl acetate, gradually heat up to 110°C over 9 hours, then heat up to 120°C~122°C in 10 hours, and finally keep warm at 120°C~122°C for reaction After 4 hours, after the reaction is complete, cool the reaction liquid to 90°C-100°C, directly add 5% sodium hydroxide solution, and alkalinize to pH=9-10. The distilled isopropyl acetate is divided into two extractions of the reaction solution, layered, and 500 g of drinking water is added to the combined organic phase for washing, layered, and the above operations are repeated until the pH value of the washing water is 7. After washing,...
Embodiment 2
[0038] The preparation of embodiment 2 hydroxychloroquine sulfate
[0039] 2.1 Preparation of hydroxychloroquine
[0040] According to embodiment 1.1 as the basis, change catalyst type, do not change the reaction effect after consumption, other conditions are constant, the result obtained is as follows:
[0041] table 2-1
[0042] sequence Catalyst type yield HPLC purity largest single impurity 1 Sodium methoxide 85.1% 99.0% <0.1% 2 Sodium tert-butoxide 85.4% 99.1% <0.1% 3 Sodium tert-amylate 86.0% 99.0% <0.1%
[0043] 2.2 Preparation of hydroxychloroquine sulfate
[0044] Based on Example 1.2, the hydroxychloroquine obtained in Example 2.1 is used to carry out the salt-forming reaction, and other conditions are constant, and the results are as follows:
[0045] Table 2-2
[0046]
Embodiment 3
[0047] The preparation of embodiment 3 hydroxychloroquine sulfate
[0048] 3.1 Preparation of hydroxychloroquine
[0049] In a three-neck round bottom flask, add 4,7-dichloroquinoline (198.0g, 1.0mol), hydroxychloroquine side chain (182.7g, 1.05mol) and tert-butyl acetate 1089g, slowly add sodium ethoxide (13.6g, 0.2mol), slowly heat up to reflux under stirring conditions, then distill off tert-butyl acetate, gradually heat up to 110°C over 9 hours, then heat up to 120°C~122°C in 10 hours, and finally keep warm at 120°C~122°C for reaction After 4 hours, after the reaction is complete, cool the reaction solution to 90°C-100°C, add 5% sodium hydroxide solution, and alkalize to pH=9-10. The distilled tert-butyl acetate was divided into two extractions of the reaction solution, layered, and 500 g of drinking water was added to the combined organic phase for washing, layered, and the above operations were repeated until the pH value of the washing water was 7. After washing, cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com