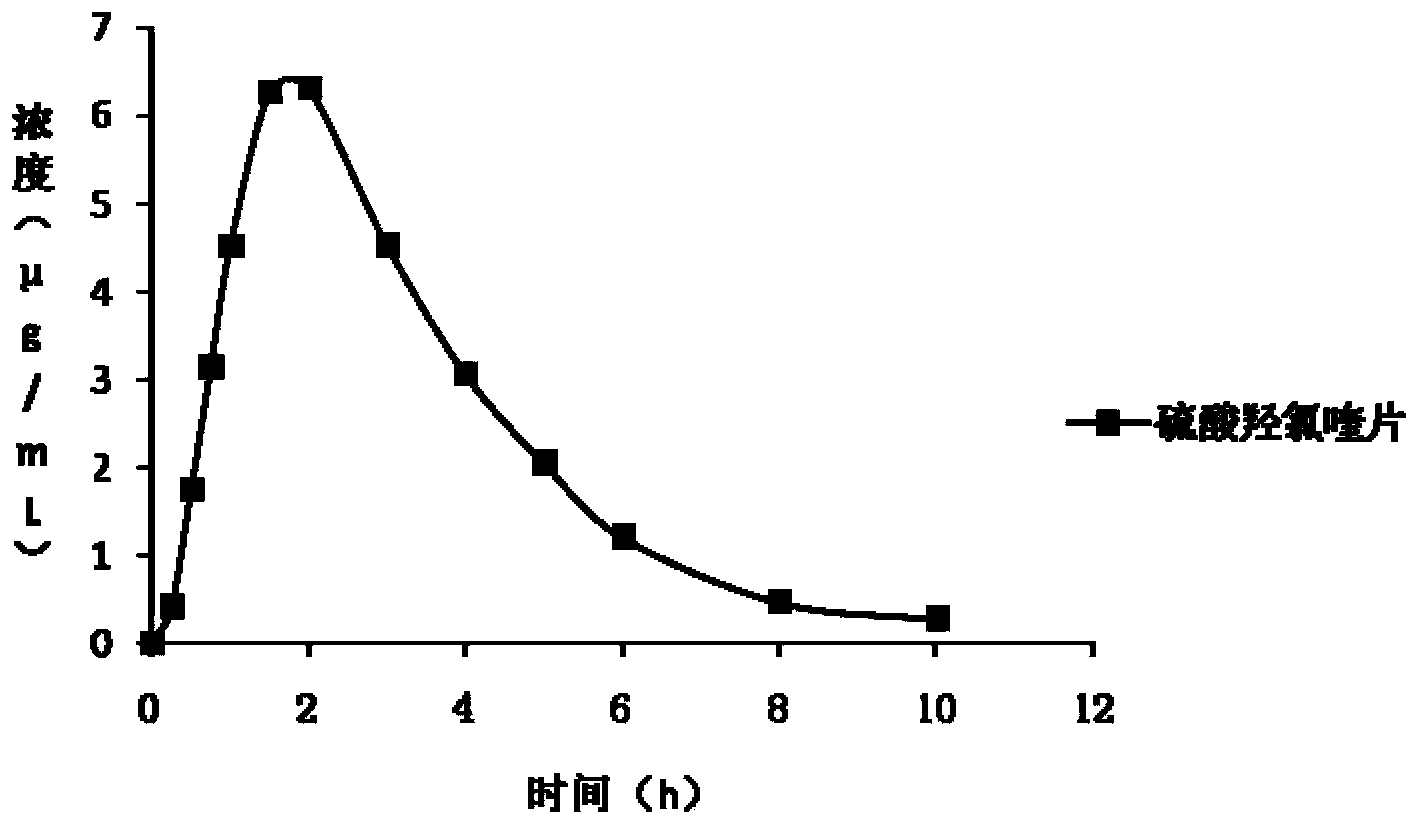
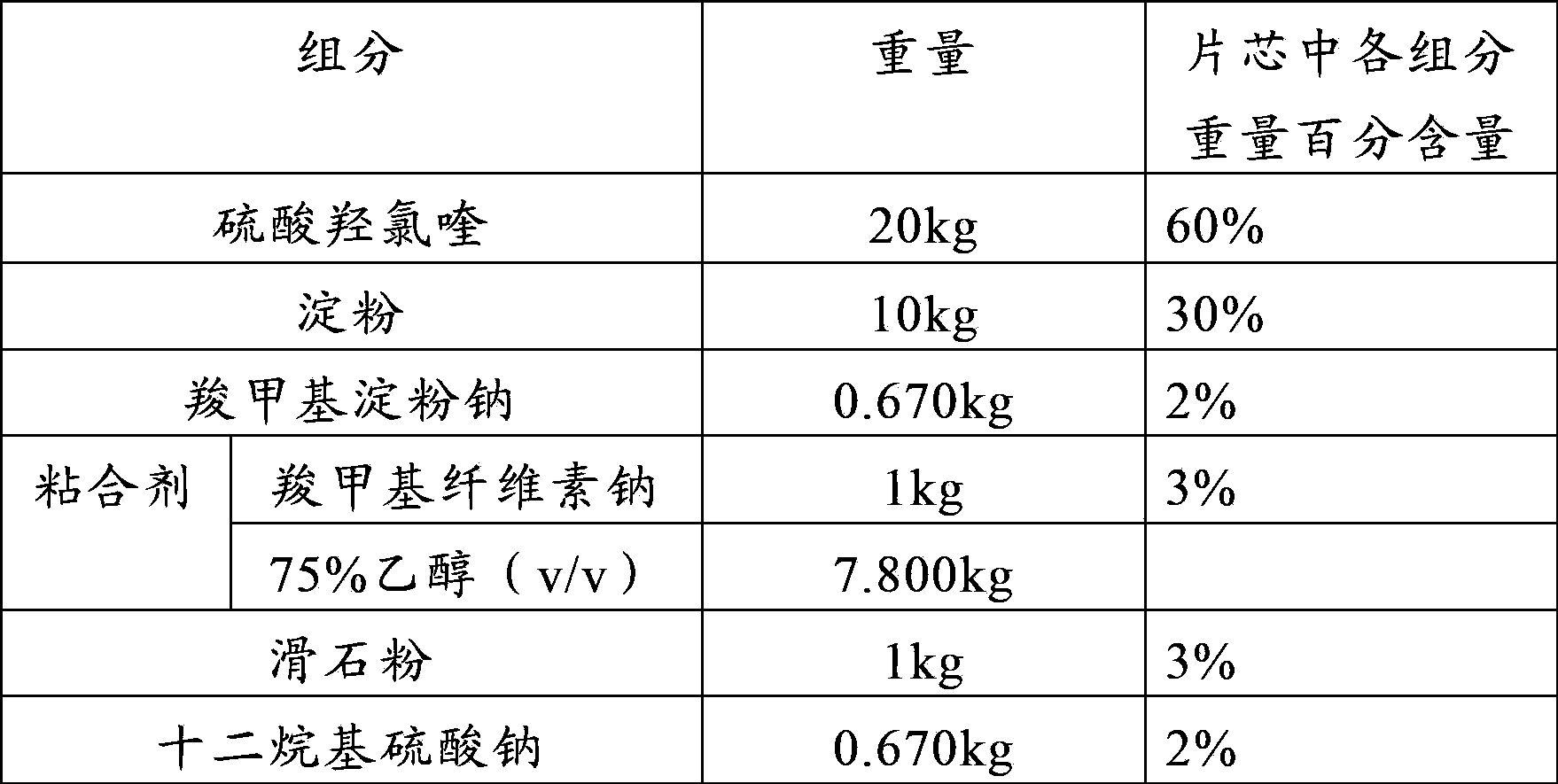
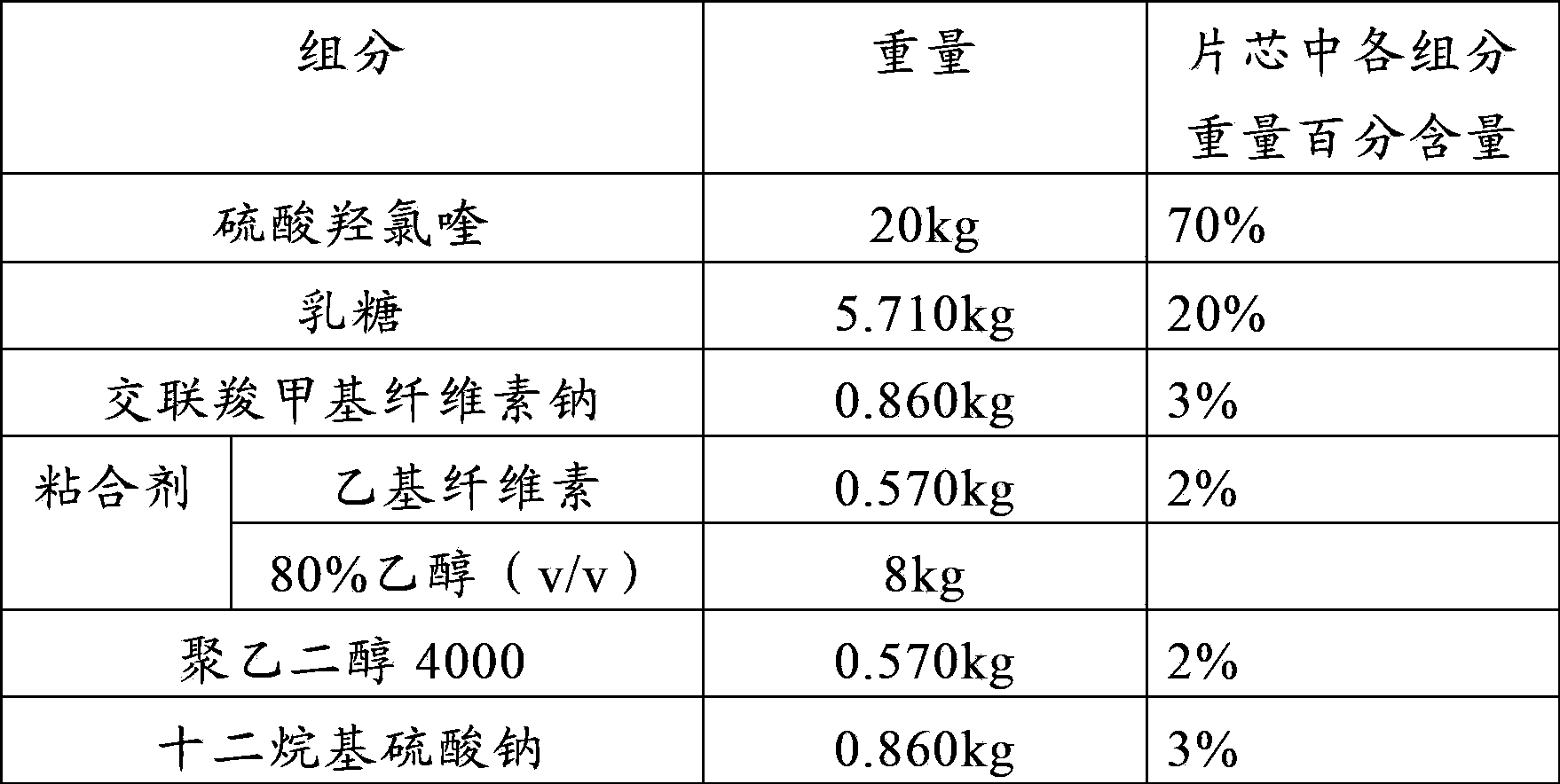
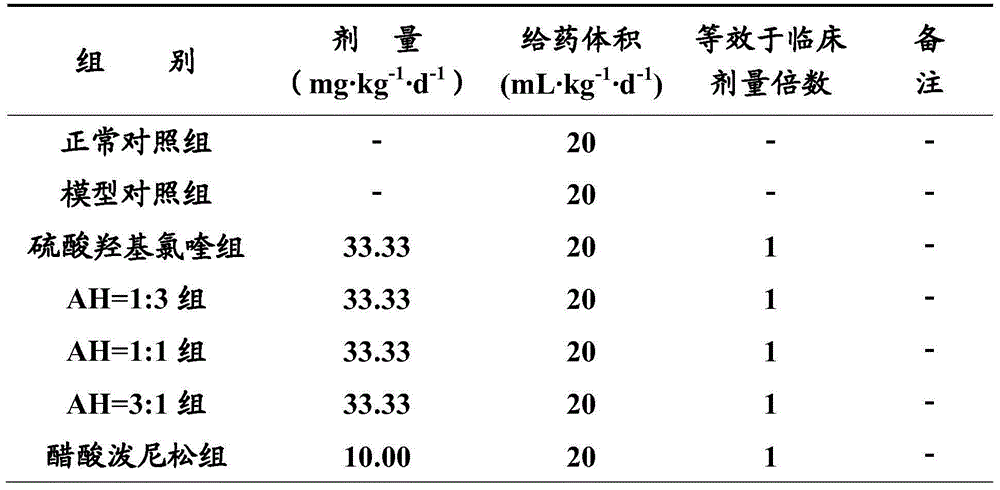
Patents
Literature
55 results about "Hydroxychloroquine Sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
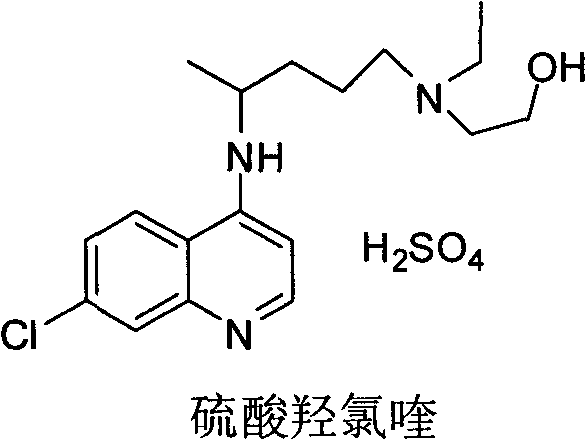
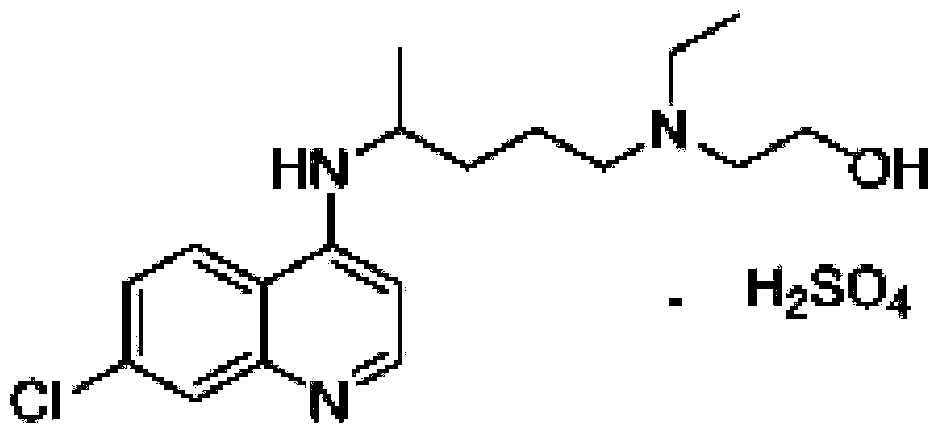
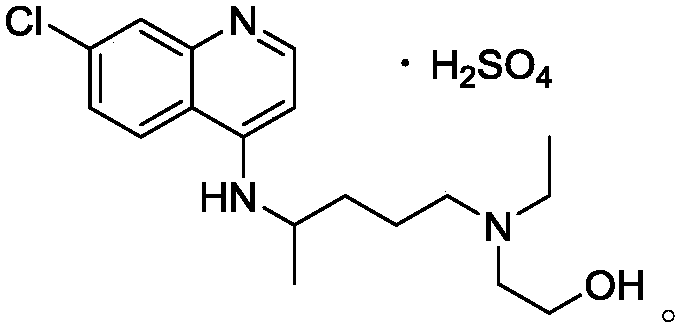
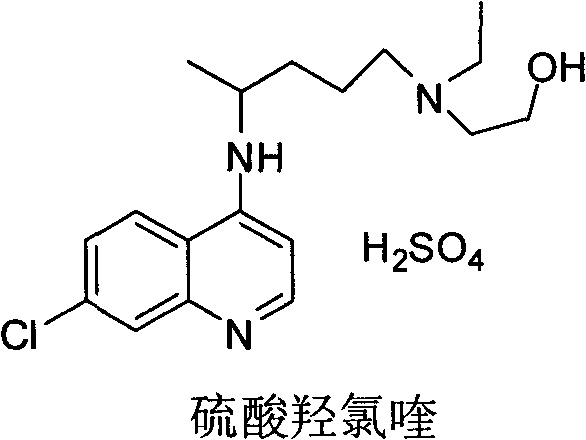
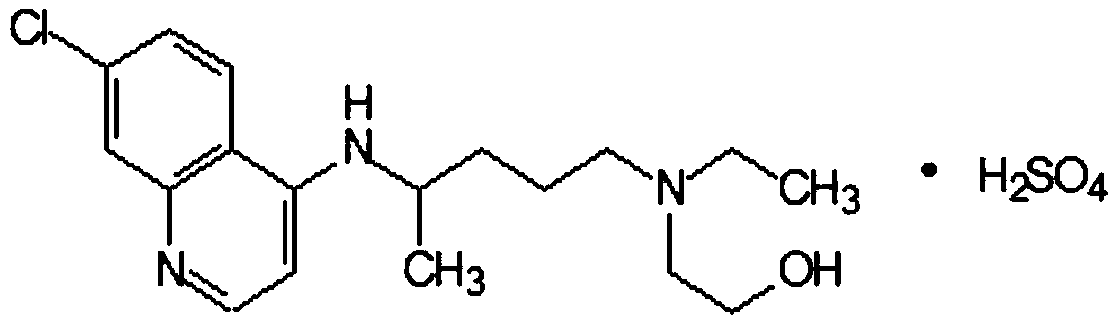
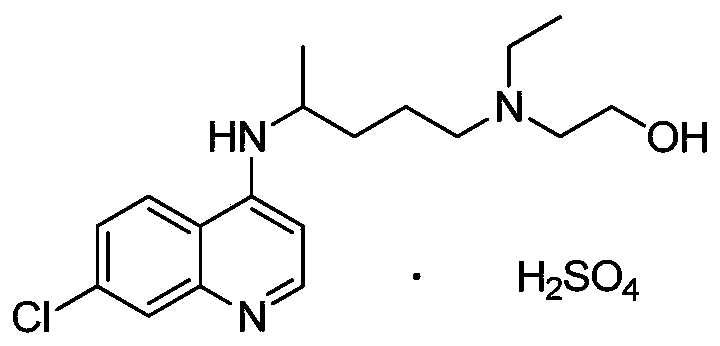
A synthetic derivative of quinolyl with chemotherapeutic and antibiotic properties, Hydroxychloroquine Sulfate acts against erythrocytic malarial parasites (Plasmodium vivax, ovale, and malariae) by concentrating in food vacuoles. It inhibits plasmodial heme polymerase and acts through other unknown mechanisms. Hydroxychloroquine also has anti-inflammatory properties and is used in the treatment of rheumatoid arthritis and lupus erythematosus. (NCI04)
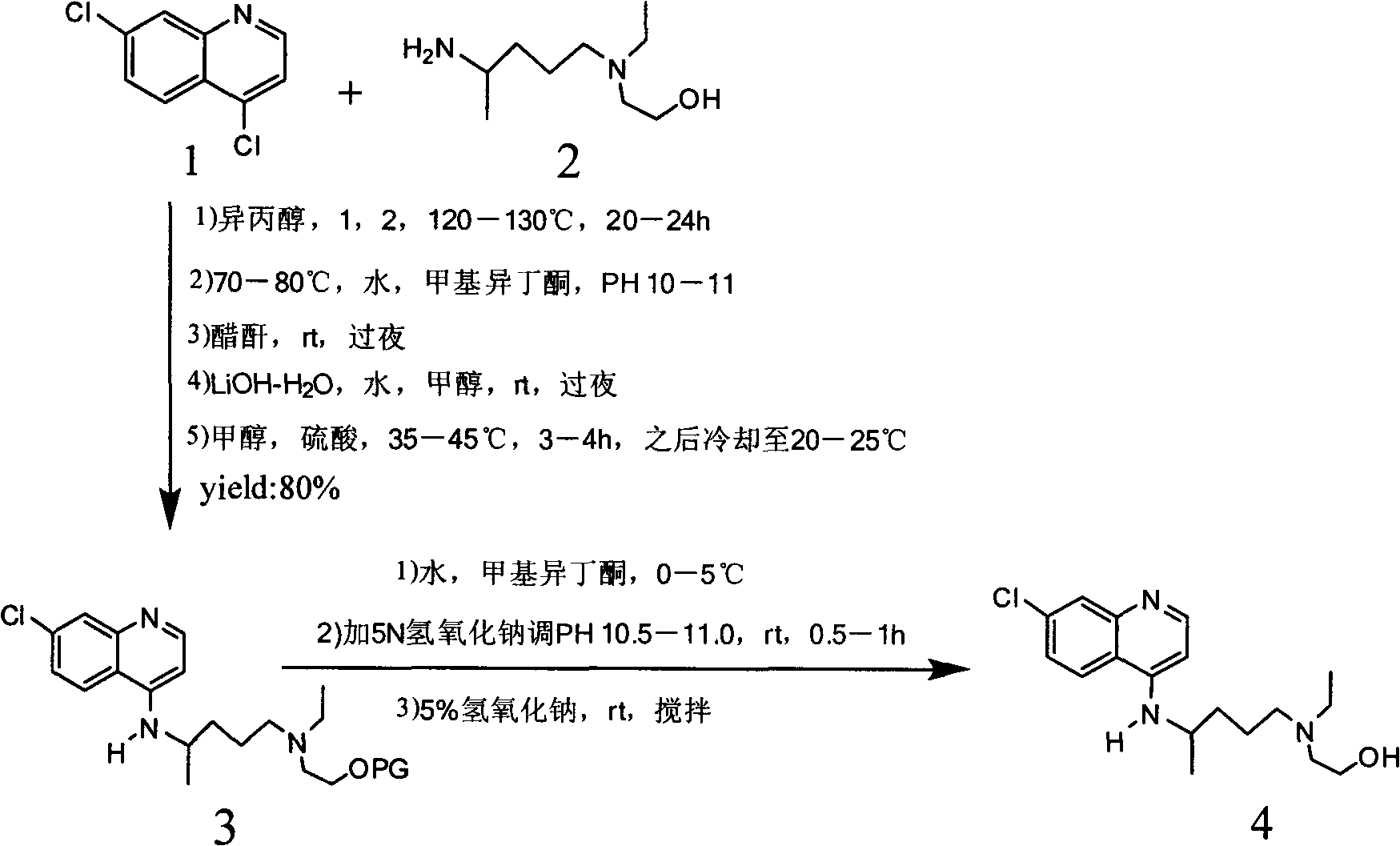
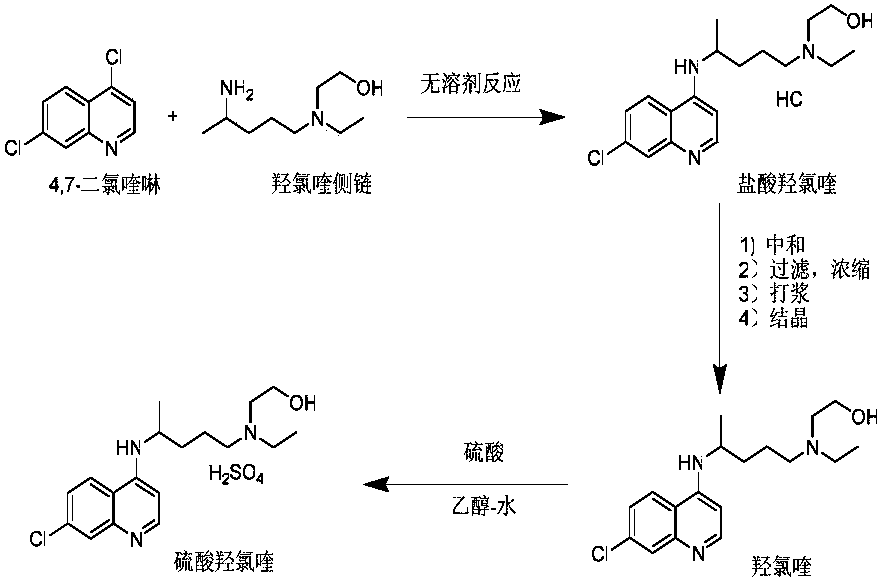
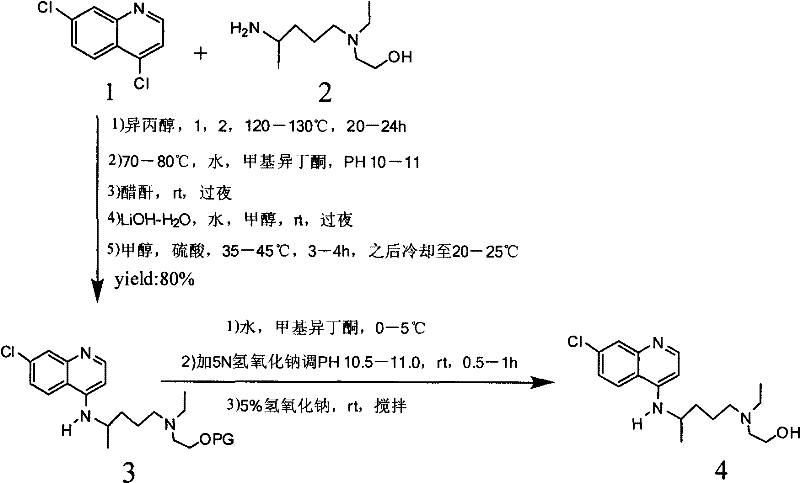
Industrial preparation method of hydroxychloroquine sulfate
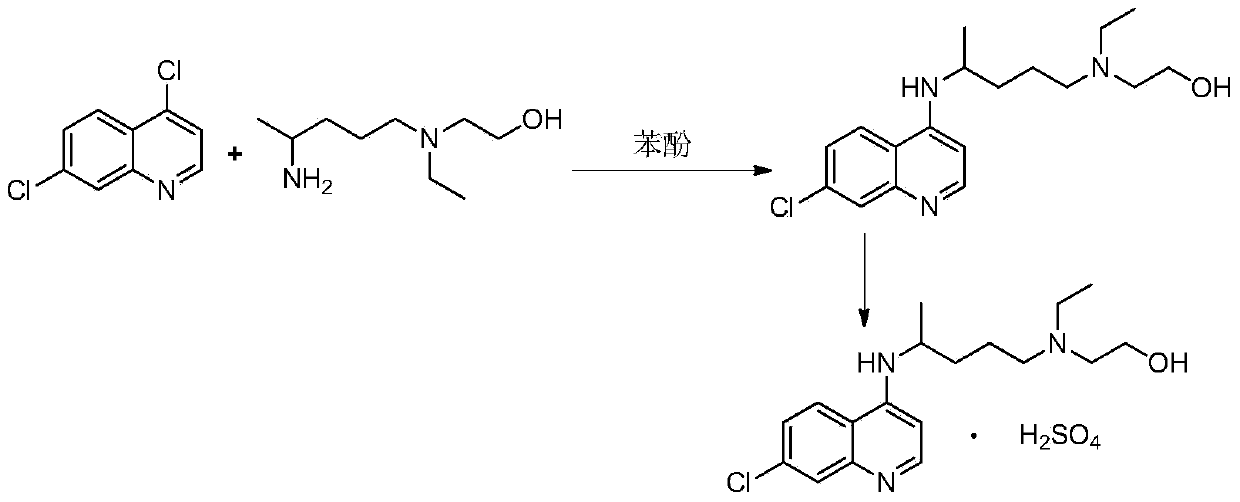
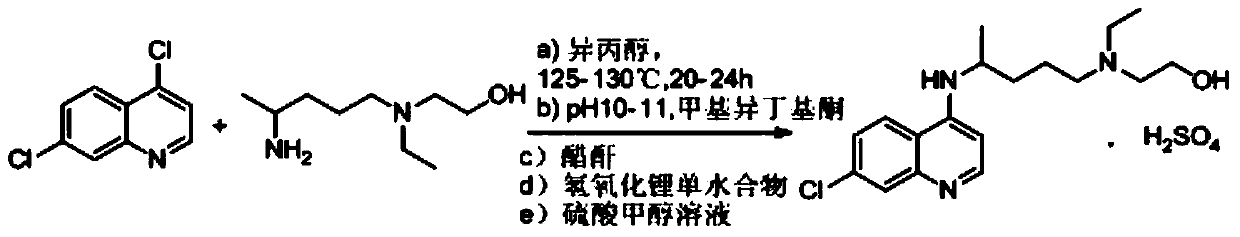
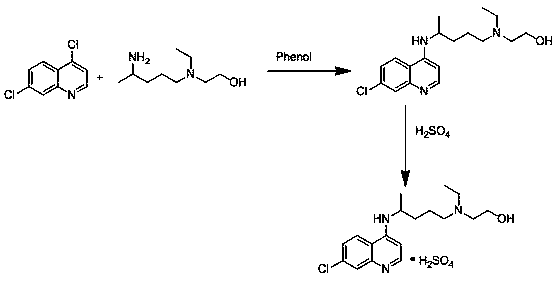
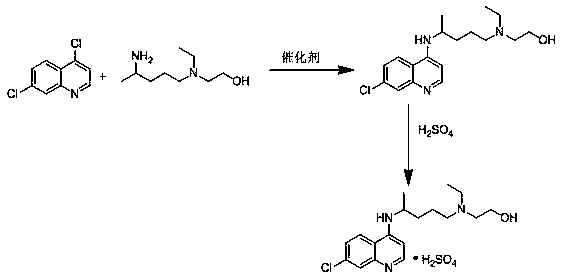
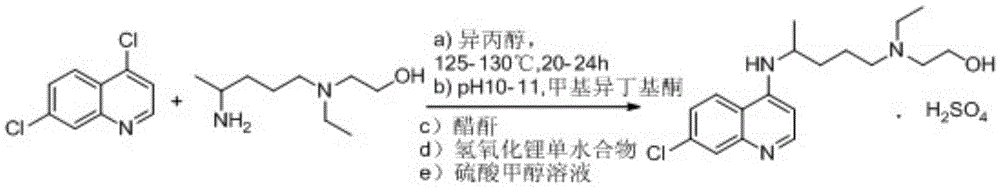
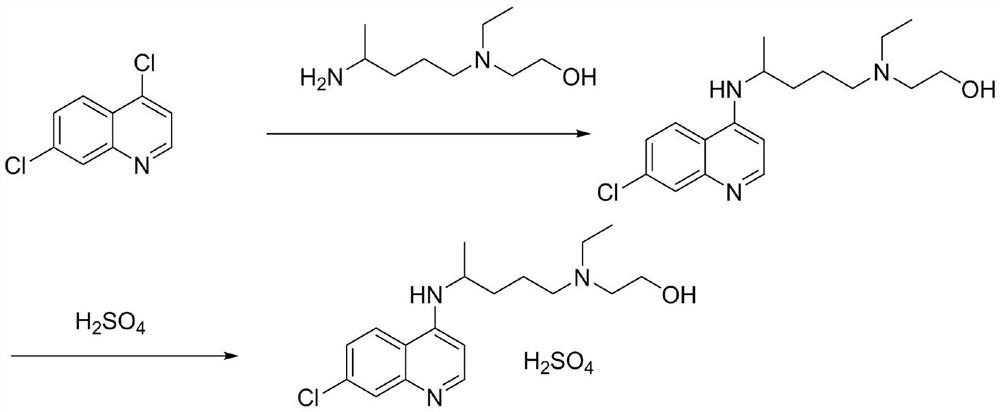
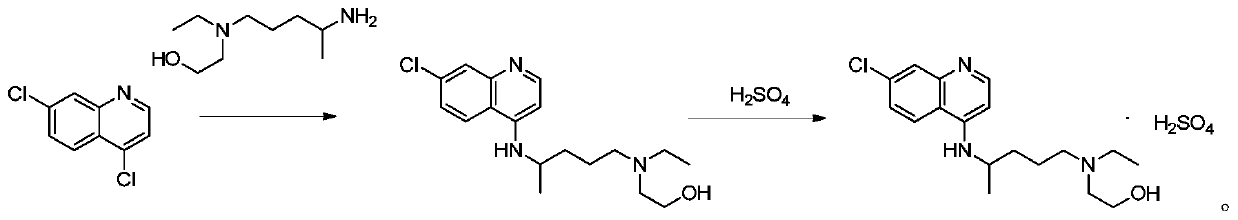
The invention relates to an industrial preparation method of hydroxychloroquine sulfate, which comprises heating 4, 7-dichloroquinoline and hydroxychloroquine side chain at refluxing temperature to 120-125 DEG C, allowing reaction to obtain hydroxychloroquine, and reacting with sulfuric acid to obtain hydroxychloroquine sulfate. The method can obtain high-purity hydroxychloroquine sulfate with single impurity less than or equal to 0.1% and purity higher than or equal to 99.5%; and has less preparation procedures, simple process, high product yield, good quality, low environmental pollution, no use of highly toxic solvent, and is easy for industrial production.
Owner:CHONGQING KANGLE PHARMA
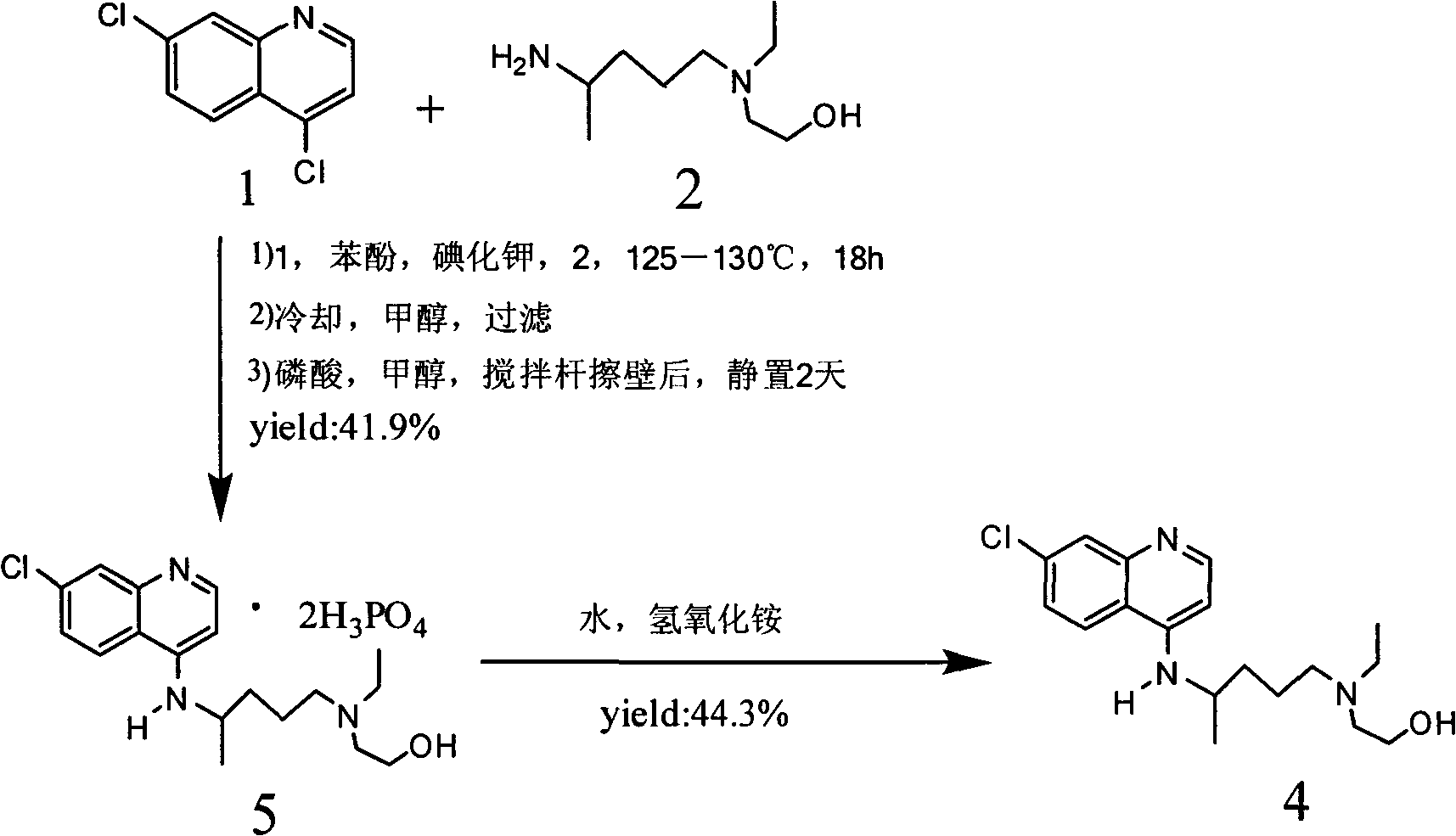
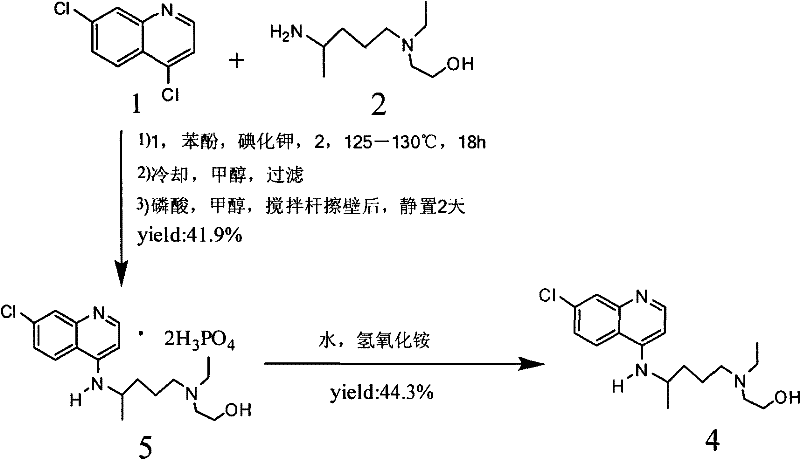
Novel industrial production method for hydroxychloroquine sulfate
The invention provides an industrial production method for hydroxychloroquine sulfate, which includes the following steps: enabling 4.7-dichloroquinoxaline and 5-(N-ethyl-N-ethoxyl)-2-amino pentane to react under gas shield for 13-24 h at a gradually increased temperature of 120-130 DEG C to obtain hydroxychloroquine; preparing the hydroxychloroquine sulfate after the reaction between the hydroxychloroquine and an alcohol sulfate solution at the temperature of 20-30 DEG C. According to the method, the yield of the obtained crude product of the hydroxychloroquine is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate HPLC is not smaller than 99.5%, the yield of single impurity is not larger than 0.1%, so that requirements of United States Pharmacopeia is met; the novel method is simple in procedure, is environment-friendly and easy in industrial production.
Owner:WUHAN WUYAO PHARMA
Novel preparation method of hydroxychloroquine sulfate
InactiveCN109456266AReduce the use effectReduce typesOrganic chemistrySide chainHydroxychloroquine Sulfate
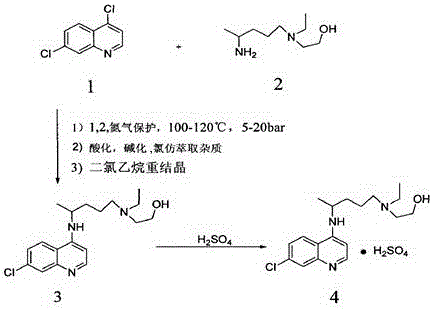
The invention discloses a preparation method of hydroxychloroquine sulfate. The preparation method is characterized in that a parent core 4,7-dichloroquinoline used as a starting material and a hydroxychloroquine side chain 5-(N-ethyl-N-2- ethanolamine)-2-amylamine undergo condensation reaction in the presence of a catalyst to obtain a hydroxychloroquine free base, and then the hydroxychloroquinefree base undergoes salt formation with sulfuric acid to obtain the hydroxychloroquine sulfate. The preparation method overcomes the disadvantages in the prior art, and has the advantages that the useamount of the side chain is reduced; the total yield is more than or equal to 90 percent; the yield of the hydroxychloroquine sulfate is more than or equal to 96 percent; the total yield is more thanor equal to 86 percent; the purity of the hydroxychloroquine sulfate is more than 99. 7 percent; and the single impurity is less than 0.1 percent. The preparation method meets the pharmacopoeia requirements and is short in reaction time, easy and convenient to operate, low in pollution, low in cost and suitable for industrial production.
Owner:南京天际联盟医药科技有限公司
Technology for preparing hydroxychloroquine sulfate tablets
ActiveCN102920674ALow hygroscopicitySimple preparation processOrganic active ingredientsAntipyreticMoisture absorptionHydroxychloroquine Sulfate
The invention discloses a technology for treating a hydroxychloroquine sulfate raw medicament. The treatment technology comprises the following steps of: performing wet granulation on the hydroxychloroquine sulfate raw medicament by using absolute ethanol as a wetting agent, and drying. The moisture absorption of the hydroxychloroquine sulfate treated by the treatment technology is obviously reduced. The invention also discloses a method for preparing the hydroxychloroquine sulfate tablets by using the hydroxychloroquine sulfate obtained by the treatment technology. The method is easy to operate, can be used for well solving the adaptability with equipment in granulation and tabletting processes, improving the reproducibility of the process and stability of the quality, and has a good commercial prospect.
Owner:湖北舒邦药业有限公司
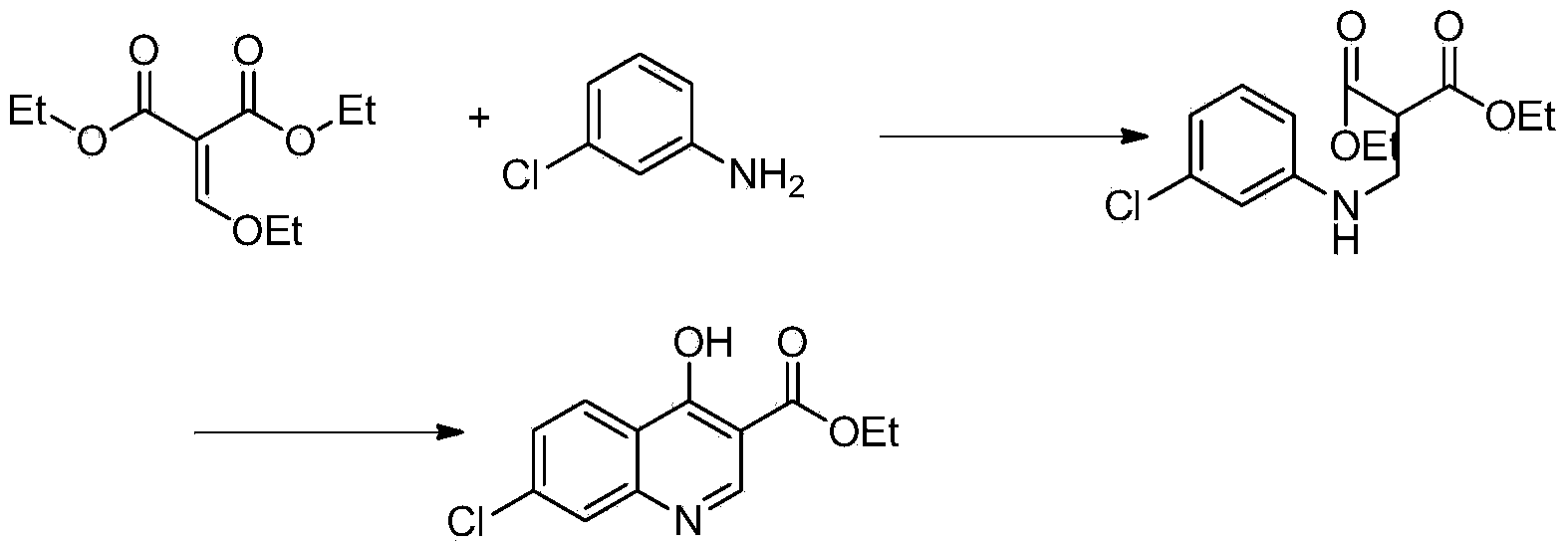
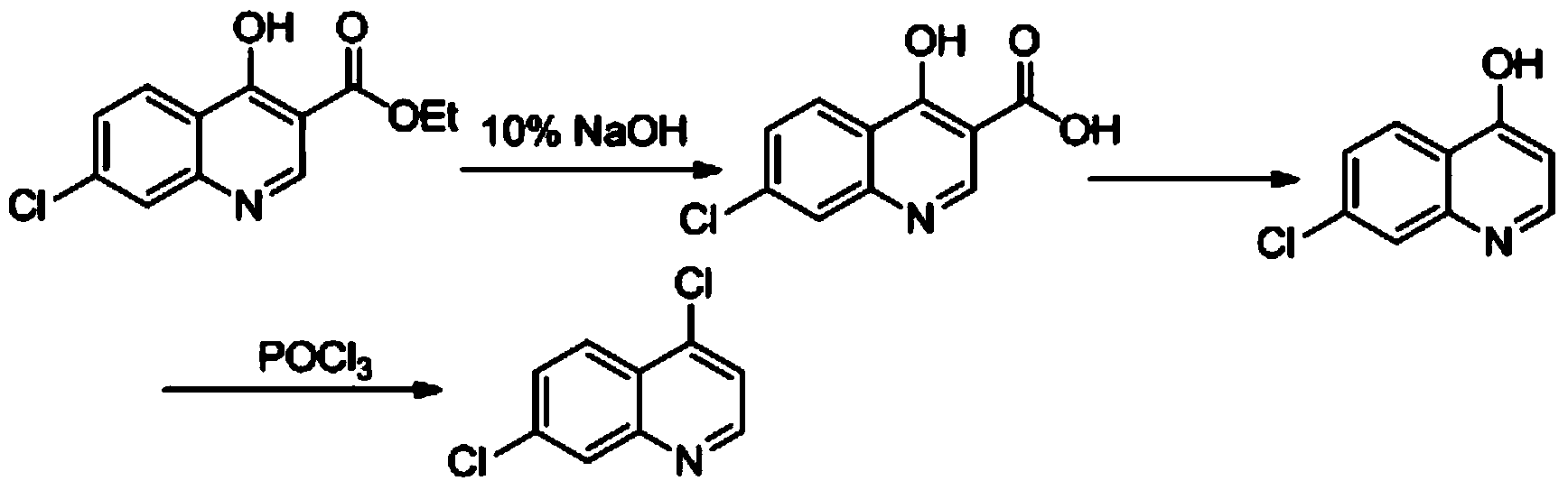
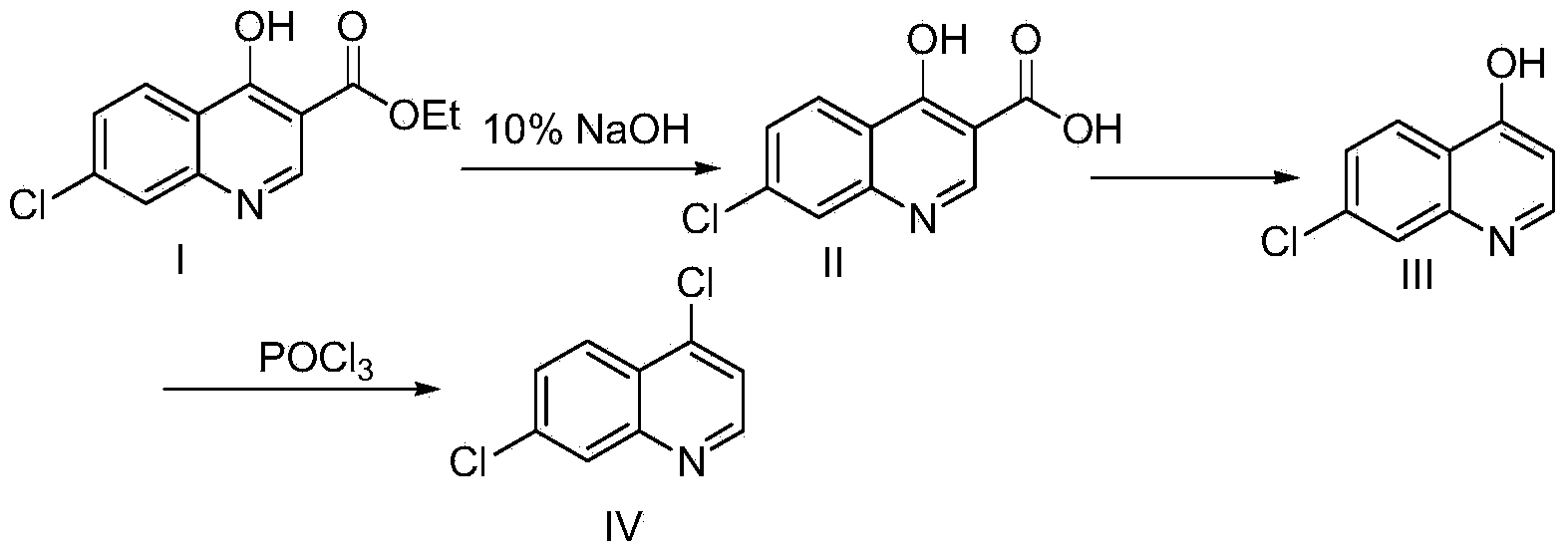
Industrial preparation method of 4,7-dichloroquinoline
The invention relates to a preparation method of a medical intermediate 4,7-dichloroquinoline. The 4,7-dichloroquinoline is an important intermediate of a medicine hydroxychloroquine sulfate for treating discoid lupus erythematosus and systemic lupus erythematosus. The preparation method comprises the following steps: performing hydrolysis and acid adjustment on 4-hydroxyl-7-chlorine-quinoline-3-carboxylic acid ethyl ester by using 10% sodium hydroxide solution to prepare 4-hydroxyl-7-chlorine-quinoline-3-carboxylic acid; performing decarboxylation to produce 4-hydroxyl-7-chloroquinoline; and chlorinating the 4-hydroxyl-7-chloroquinoline by using phosphorus oxychloride to obtain 4,7-dichloroquinoline crude products; and performing one-step refining to obtain the products. The purity of the prepared products is more than or equal to 99% and the total yield of the products is more than or equal to 70%; raw materials are easily available; the process is simple; the yield and the purity are high in each step; and the preparation method is suitable for industrialized production.
Owner:WUHAN WUYAO PHARMA
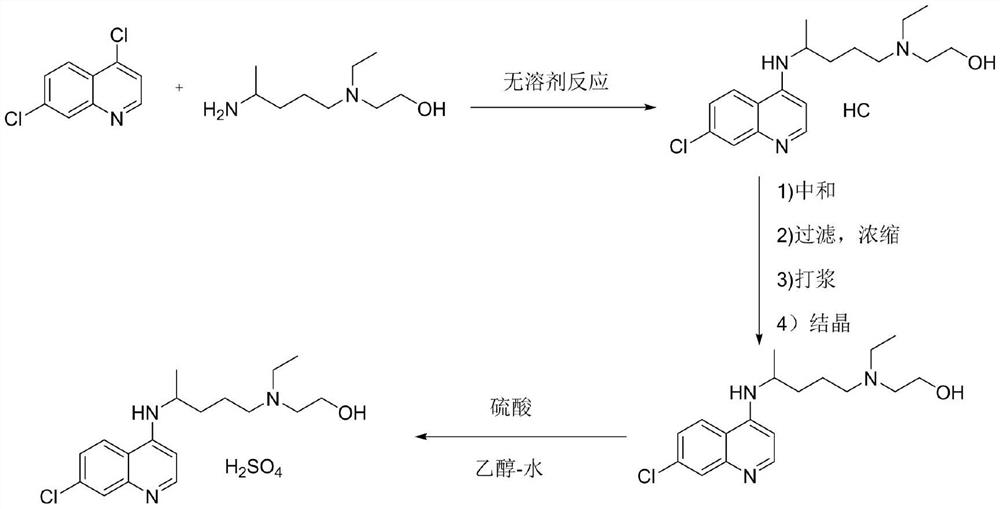
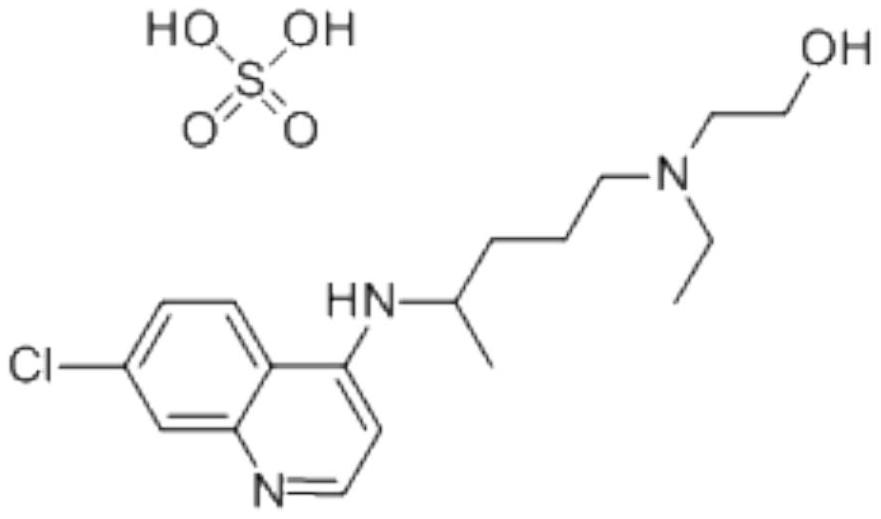
Preparation method of hydroxychloroquine sulfate
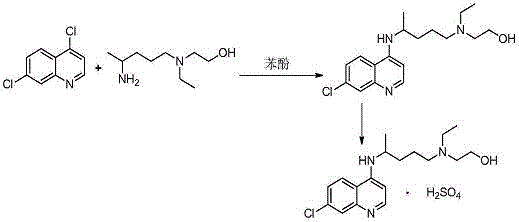
The invention discloses a preparation method of high-purity hydroxychloroquine sulfate. The method uses 4,7-dichloroquinoline and hydroxychloroquine side chain as raw materials to directly prepare hydroxychloroquine hydrochloride, hydroxychloroquine hydrochloride is neutralized with sodium alcoholate or potassium alcoholate, filtered, concentrated, beaten and crystallized to obtain hydroxychloroquine refined product, and the hydroxychloroquine refined product finally is subjected to salifying reaction with sulfuric acid in a certain proportion of pure aqueous solution to obtain hydroxychloroquine sulfate. The method avoids the use of phenol or its catalyst in the process of preparing hydroxychloroquine, avoids the extraction operation in the post-treatment, has high product purity, and basically does not generate waste water in the production process. The method is convenient to operate and has high yield, the HPLC purity of prepared hydroxychloroquine sulfate is more than or equal to99.6%, and maximum single impurity is less than or equal to 0.1%, and that method is more suitable for industrial production.
Owner:SHANGHAI INST OF TECH
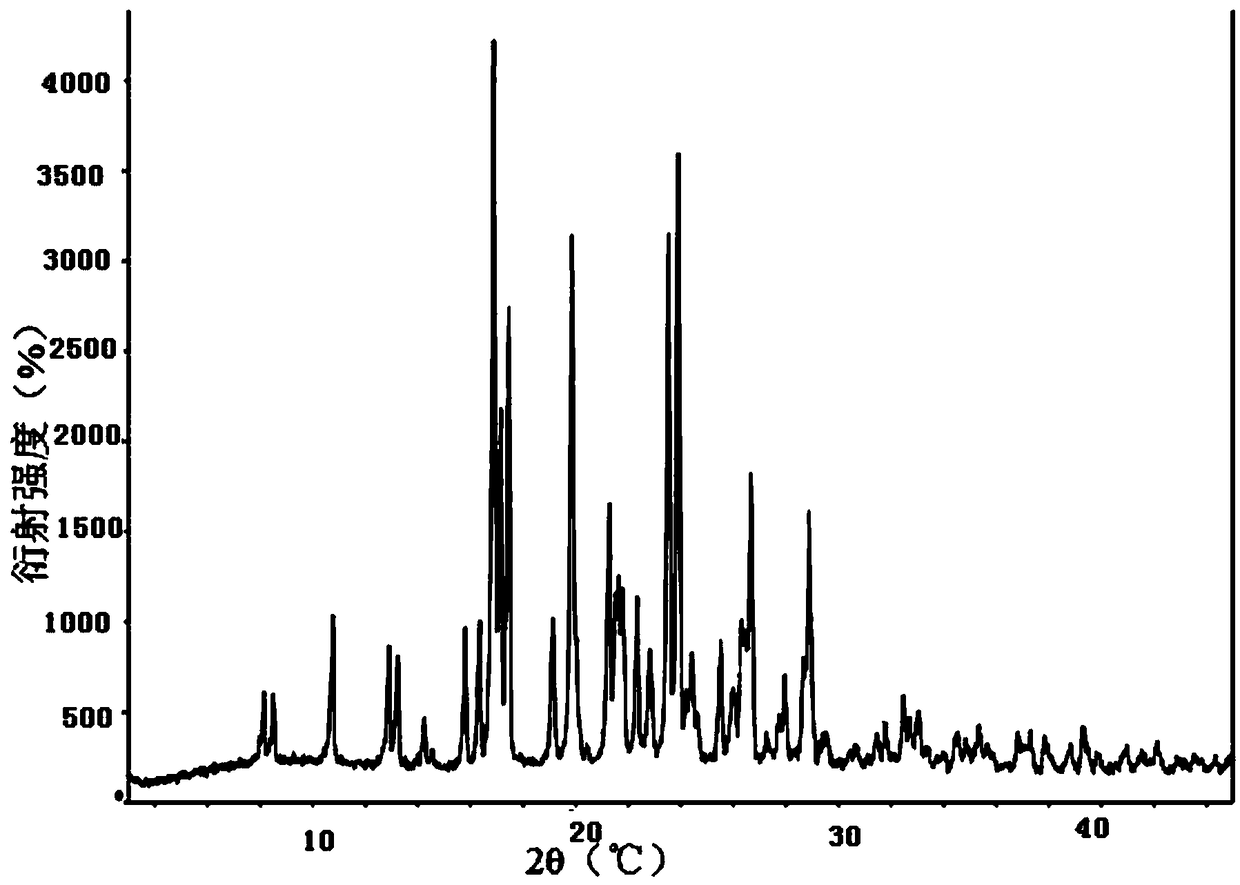
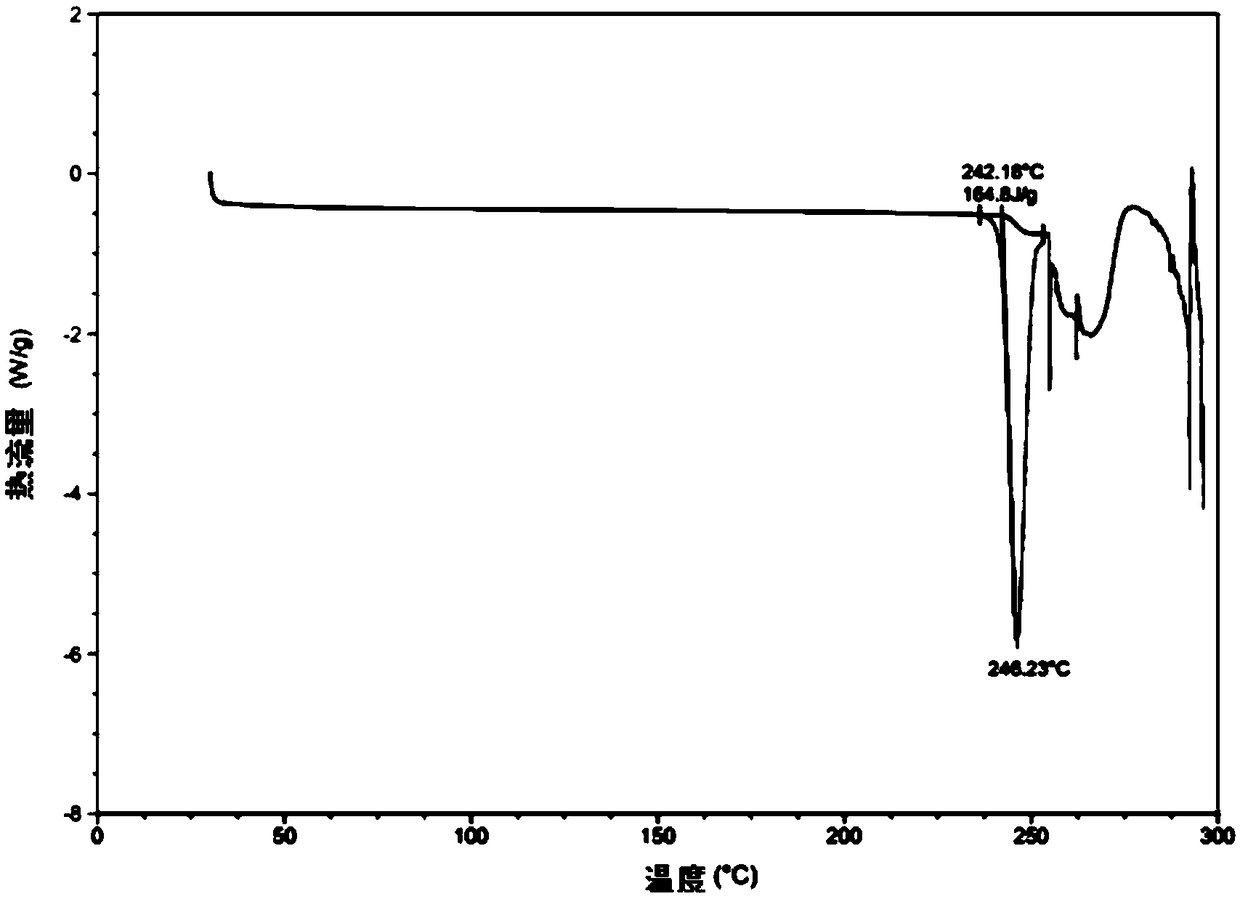
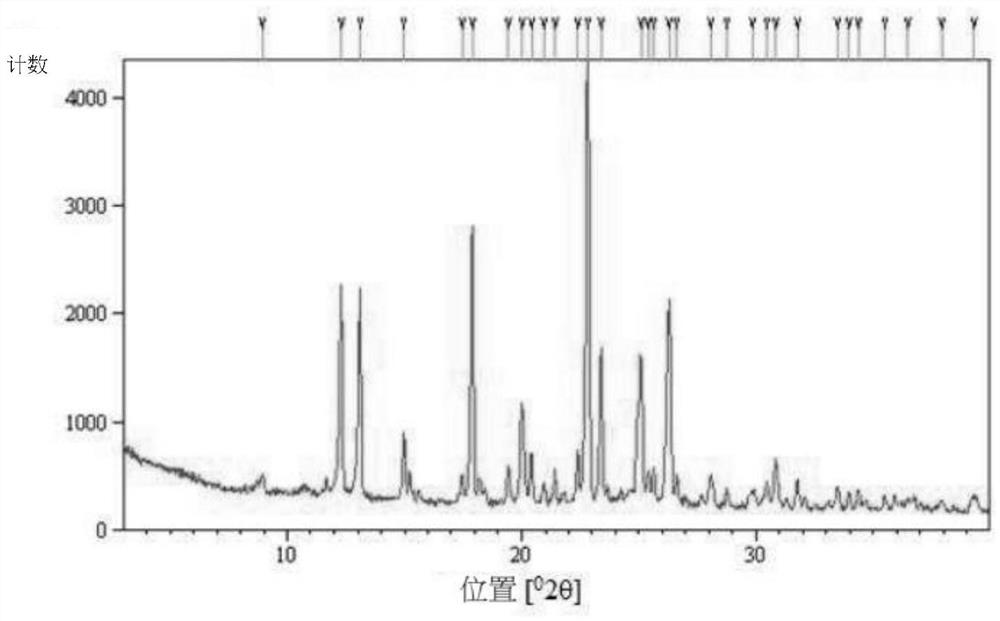
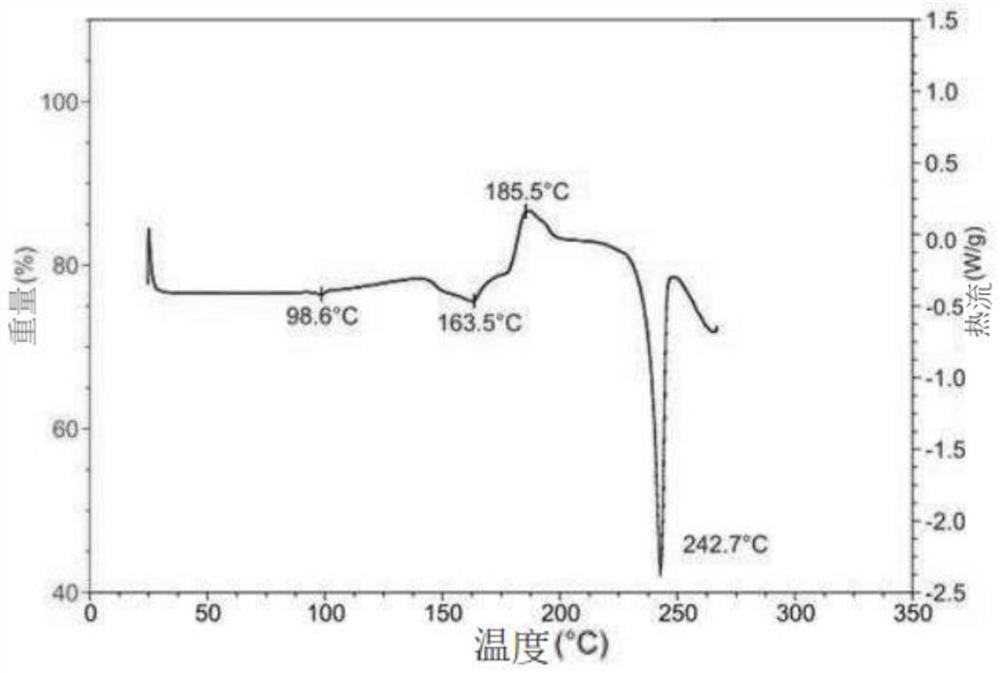
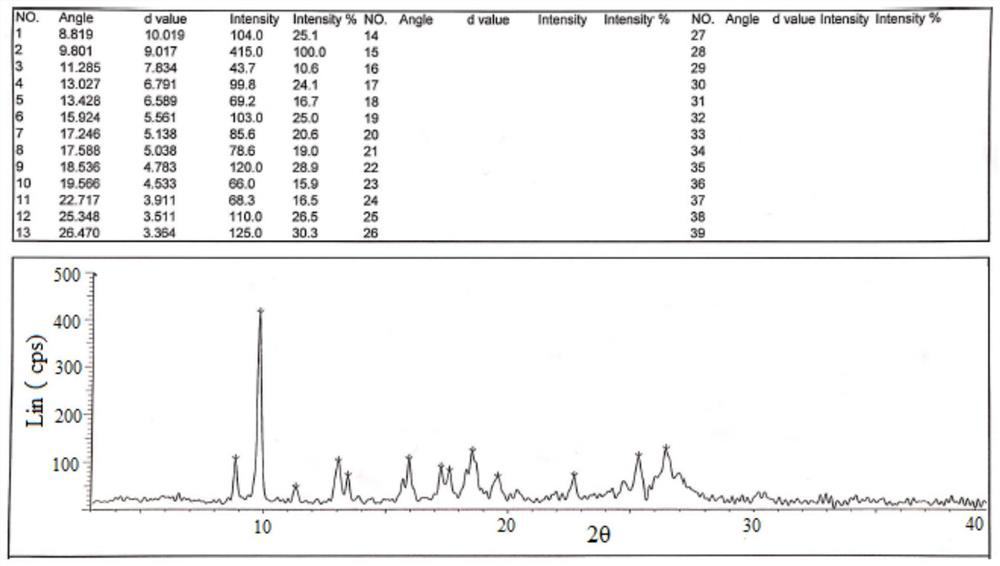
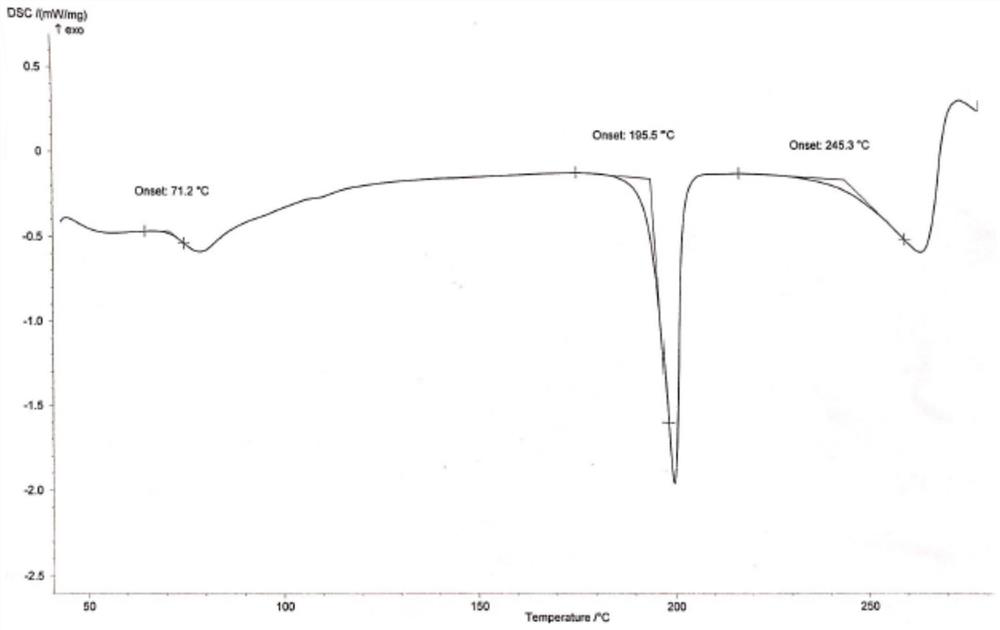
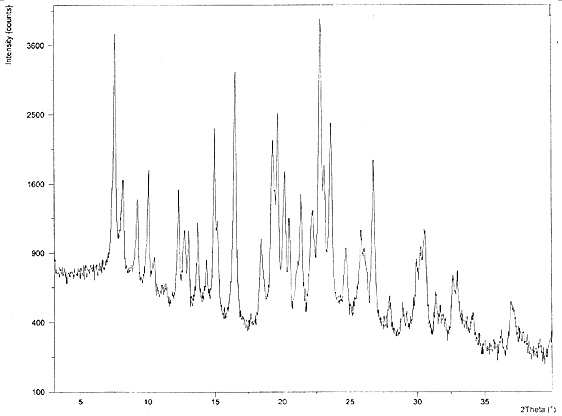
Hydroxychloroquine sulfate crystal form A and preparation method thereof
InactiveCN108727263AGood chemical stabilityEase of industrial productionOrganic chemistry methodsHydroxychloroquine SulfateAqueous solution
The invention discloses a hydroxychloroquine sulfate crystal form A and a preparation method thereof and uses means of XRPD, DSC and IR for representation. The hydroxychloroquine sulfate crystal formA provided by the invention improves the chemical stability of hydroxychloroquine sulfate, is simple in preparation method and easy in industrialized production and can provide a study foundation to development of more hydroxychloroquine sulfate dosage forms at the same time. In the preparation method provided by the invention, a sulfuric acid aqueous solution with the mass percentage being 40-60percent is used, the safety and the operability are improved, and the heat release risk generated by using concentrated sulfuric acid is effectively lowered.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
Application of artesunate as drug for treating systemic lupus erythematosus
InactiveCN101632657AMature production processQuality improvementOrganic active ingredientsSkeletal disorderLupus pernioSide effect
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
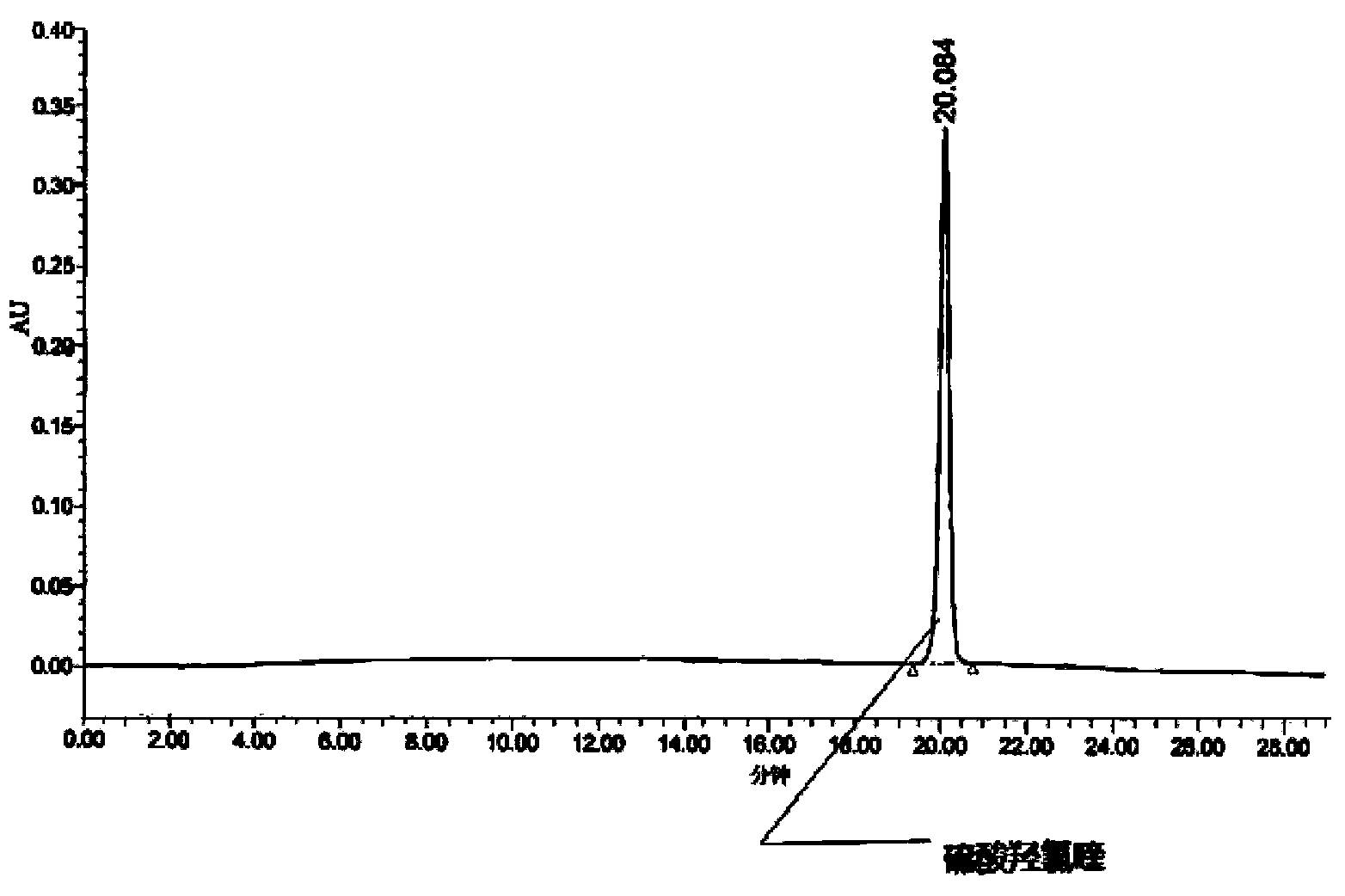
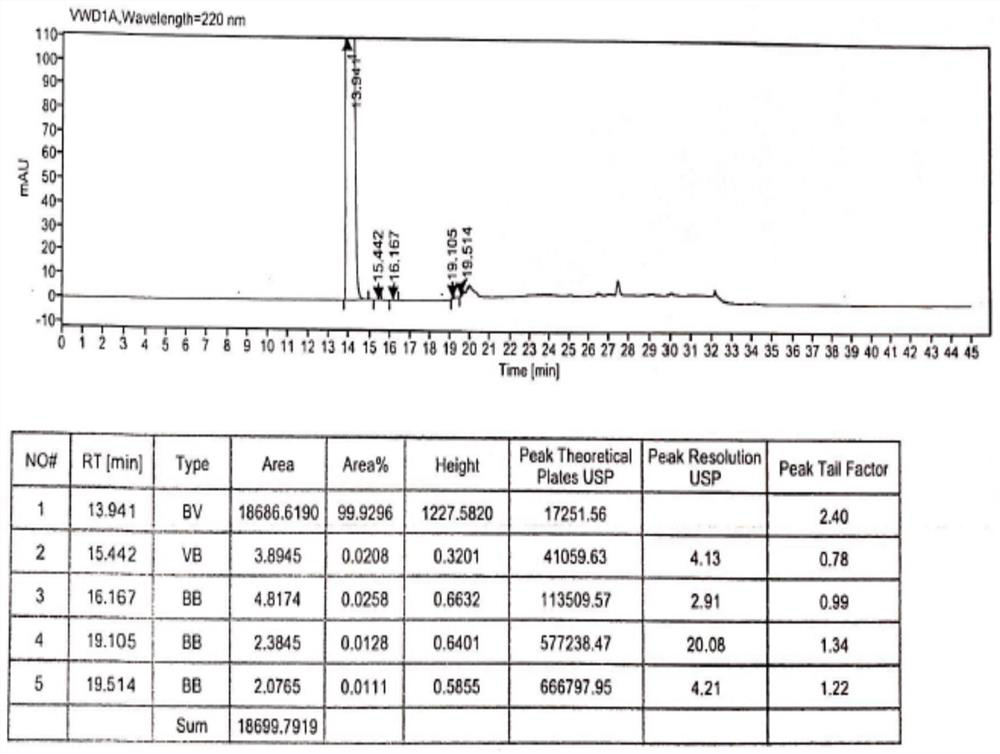
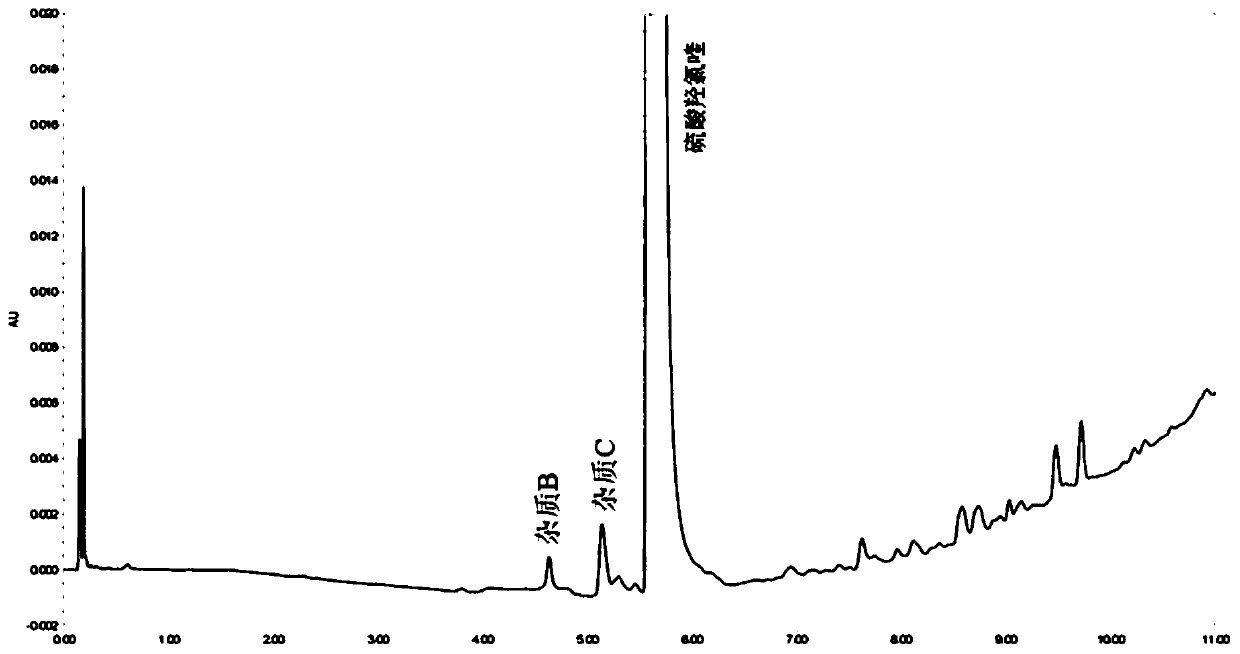
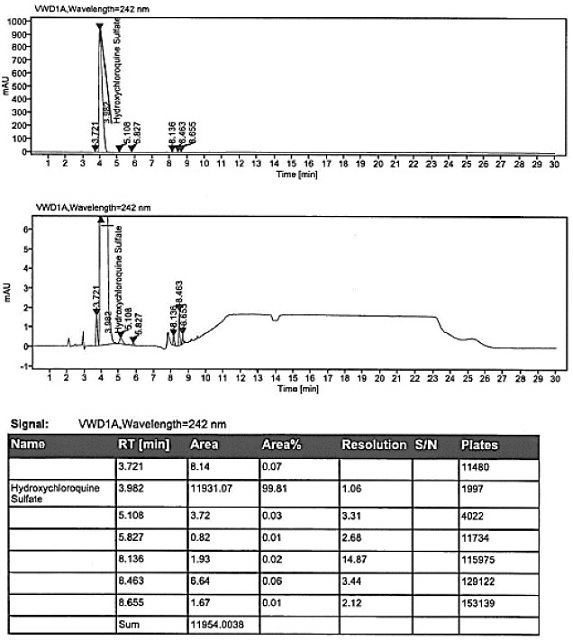
Method for analysis of hydroxychloroquine sulfate raw material and preparation by high performance liquid chromatography
InactiveCN103472154AOvercoming the lack of specificityOvercoming sensitivityComponent separationHydroxychloroquine SulfateHigh-performance liquid chromatography
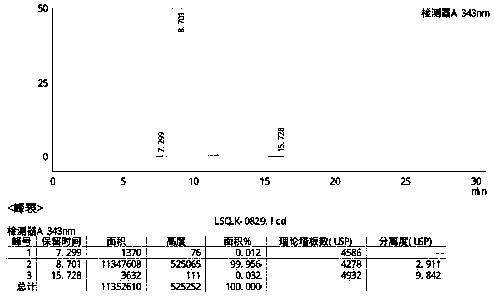
The invention belongs to the field of drug analysis and discloses a method for analysis of a hydroxychloroquine sulfate raw material and preparation by high performance liquid chromatography. The method can realize effective separation of related substances in hydroxychloroquine sulfate so that hydroxychloroquine sulfate quality is controlled, hydroxychloroquine sulfate raw material and preparation content is accurately determined, and hydroxychloroquine sulfate stability is indicated. The method has the advantages of strong specificity, high accuracy and simple operation.
Owner:WUHAN WUYAO SCI & TECH
Industrial preparation method of hydroxychloroquine sulfate
The invention relates to an industrial preparation method of hydroxychloroquine sulfate, which comprises heating 4, 7-dichloroquinoline and hydroxychloroquine side chain at refluxing temperature to 120-125 DEG C, allowing reaction to obtain hydroxychloroquine, and reacting with sulfuric acid to obtain hydroxychloroquine sulfate. The method can obtain high-purity hydroxychloroquine sulfate with single impurity less than or equal to 0.1% and purity higher than or equal to 99.5%; and has less preparation procedures, simple process, high product yield, good quality, low environmental pollution, no use of highly toxic solvent, and is easy for industrial production.
Owner:CHONGQING KANGLE PHARMA
Technology for preparing hydroxychloroquine sulfate tablets
ActiveCN102920674BSimple preparation processLow hygroscopicityOrganic active ingredientsAntipyreticHydroxychloroquine SulfateMoisture absorption
The invention discloses a technology for treating a hydroxychloroquine sulfate raw medicament. The treatment technology comprises the following steps of: performing wet granulation on the hydroxychloroquine sulfate raw medicament by using absolute ethanol as a wetting agent, and drying. The moisture absorption of the hydroxychloroquine sulfate treated by the treatment technology is obviously reduced. The invention also discloses a method for preparing the hydroxychloroquine sulfate tablets by using the hydroxychloroquine sulfate obtained by the treatment technology. The method is easy to operate, can be used for well solving the adaptability with equipment in granulation and tabletting processes, improving the reproducibility of the process and stability of the quality, and has a good commercial prospect.
Owner:湖北舒邦药业有限公司
Combined drug and applications of combined drug as immuno-regulation agent
ActiveCN105250295AReduce usageSmall toxicityOrganic active ingredientsAntipyreticImmunologic disordersDisease
The present invention provides a combined drug, which contains a first active ingredient artemisinin, a second active ingredient hydroxychloroquine sulfate, and an optionally pharmaceutically-acceptable excipient. According to the present invention, the combined drug can be effectively used for autoimmune disease treatment, and can reduce the hydroxychloroquine sulfate consumption under the premise of assurance of the unchanged efficacy even improvement of the efficacy so as to reduce the toxic-side effect of the hydroxychloroquine sulfate.
Owner:INST OF SCI & TECH GUANGDONG UNIV OF CHINESE MEDICINE
A kind of industrialized preparation method of hydroxychloroquine sulfate
The invention provides an industrial production method for hydroxychloroquine sulfate, which includes the following steps: enabling 4.7-dichloroquinoxaline and 5-(N-ethyl-N-ethoxyl)-2-amino pentane to react under gas shield for 13-24 h at a gradually increased temperature of 120-130 DEG C to obtain hydroxychloroquine; preparing the hydroxychloroquine sulfate after the reaction between the hydroxychloroquine and an alcohol sulfate solution at the temperature of 20-30 DEG C. According to the method, the yield of the obtained crude product of the hydroxychloroquine is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate is not smaller than 85%, the yield of the obtained hydroxychloroquine sulfate HPLC is not smaller than 99.5%, the yield of single impurity is not larger than 0.1%, so that requirements of United States Pharmacopeia is met; the novel method is simple in procedure, is environment-friendly and easy in industrial production.
Owner:WUHAN WUYAO PHARMA
A kind of preparation method of hydroxychloroquine sulfate
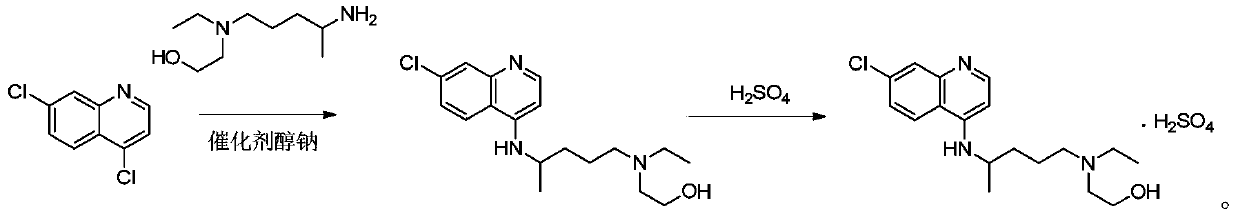
The invention discloses a preparation method of hydroxychloroquine sulfate. The preparation method is characterized by comprising the following steps: condensing 4,7-dichloroquinoline serving as an initial raw material and a hydroxychloroquine side chain under the action of a catalyst, so as to obtain hydroxychloroquine; and reacting hydroxychloroquine with sulfuric acid, so as to prepare the hydroxychloroquine sulfate. According to the method disclosed by the invention, the defects in the prior art are overcome. The method has the advantages that the yield of the prepared hydroxychloroquine sulfate crude product is greater than or equal to 85%, the yield of hydroxychloroquine sulfate is greater than or equal to 94%; the total yield is greater than or equal to 80%; the purity of the prepared hydroxychloroquine sulfate HPLC is greater than or equal to 99.6%; the maximum single impurity is smaller than 0.1%; the method accords with the requirements of United States pharmacopeia, is short in reaction step, and simple in the whole technological operation; and the obtained product is high in quality, high in yield, and relatively suitable for industrial production.
Owner:TSINGHUA TONGFANG CO LTD
Crystals of hydroxychloroquine sulfate
PendingCN113527202AOrganic active ingredientsOrganic chemistry methodsHydroxychloroquine SulfateCondensed matter physics
Two crystals of (S)-(+)-hydroxychloroquine sulfate. One crystal features diffraction peaks at 12.3 + / - 0.1 degrees, 13.1 + / - 0.1 degrees, 17.9 + / - 0.1 degrees, 22.8 + 0.1 degrees, 23.4 + 0.1 degrees, 25.1 + / - 0.1 degrees, and 26.3 + / - 0.1 degrees as 2theta angles in a powder X-ray diffraction pattern. The other crystal features diffraction peaks at 12.8 + / - 0.1 degrees, 14.5 + / - 0.1 degrees, 16.7 + 0.1 degrees, 17.6 + 0.1 degrees, 20.2 + / - 0.1 degrees, 21.4 + / - 0.1 degrees, 23.8 + / - 0.1 degrees, 25.7 + / - 0.1 degrees, and 26.0 + / - 0.1 degrees as 2theta angles in a powder X-ray diffraction pattern. Also disclosed are methods of preparing crystals of (S)-(+)-hydroxychloroquine sulfate.
Owner:GENELABS TECH INC
Method for analyzing composition of hydroxychloroquine sulfate preparation by high performance liquid chromatography
PendingCN111398475AImprove accuracyEasy to separateComponent separationHydroxychloroquineHydroxychloroquine Sulfate
The invention discloses a method for analyzing a composition of a hydroxychloroquine sulfate preparation by high performance liquid chromatography. The method comprises the following steps: a. selecting 50mg of hydroxychloroquine sulfate reference substance, and precisely weighing; b, precisely weighing 1.0 ml, 2.0 ml, 3.0 ml, 4.0 ml, 5.0 ml, 6.0 ml and 7.0 ml of the mixed solutions obtained in the step a, and putting the mixed solutions into a 10ml volumetric flask for later use; c, performing chromatographic analysis by using a chromatographic column to obtain a regression equation of a concentration C and a peak area A; and d, removing a proper amount of a mixture obtained in the step a, precisely weighing, adding a diluent to dissolve, and quantitatively diluting to prepare a solutioncontaining 0.5 mg per 1ml to obtain a test solution; and precisely measuring the test solution, adding a mobile phase, diluting to prepare a solution containing 0.05 g per 1ml as a control solution, precisely measuring 0.2 ml of the control solution, injecting the control solution into a liquid chromatograph, and adjusting sensitivity of an instrument for testing. The method for analyzing the composition of the hydroxychloroquine sulfate preparation by the high performance liquid chromatography is simple and convenient to operate, sensitive, high in accuracy, suitable for clinical hydroxychloroquine serum drug concentration monitoring and high in specificity.
Owner:NANJING MEIRUI PHARMA CO LTD
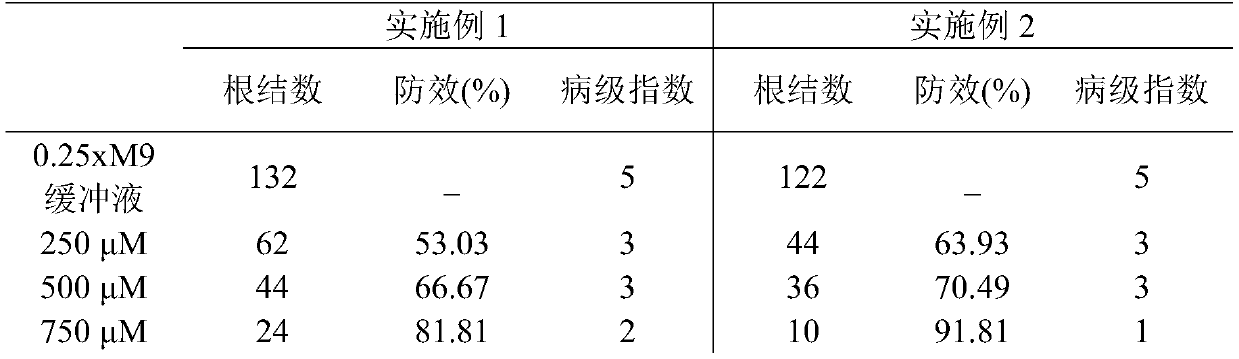
New use of chloroquine in prevention and control of root-knot nematodes
The invention discloses a new use of chloroquine in prevention and control of root-knot nematodes, and belongs to the technical field of the prevention and control of root-knot nematodes. The invention relates to a use of chloroquine in prevention and control of root-knot nematodes and a use of chloroquine in inhibition of infection, development and / or oviposition of root-knot nematodes, wherein the chloroquine is chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate. The invention also relates to a use of chloroquine in preparation of a root-knot nematode pesticide or a root-knot nematode infection, development and / or oviposition inhibitor, wherein the active component of the root-knot nematode pesticide or the root-knot nematode infection, development and / or oviposition inhibitor is chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate; and the root-knot nematode pesticide or the root-knot nematode infection, development and / or oviposition inhibitor is an aqueous solution of chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate. According to the present invention, the prevention and control of root-knot nematodes with the aqueous solution of chloroquine are safe, non-toxic and effective.
Owner:YUNNAN UNIV
Hydroxychloroquine sulfate and polyglutamic acid polymer, as well as preparation method and application thereof
InactiveCN105924641AHigh activityGood sustained release effectOrganic active ingredientsSkeletal disorderCross-linkSide effect
The invention discloses a hydroxychloroquine sulfate and polyglutamic acid polymer, as well as a preparation method and application thereof. The hydroxychloroquine sulfate and polyglutamic acid polymer is a polymer formed by carrying out esterification reaction between hydroxychloroquine sulfate and polyglutamic acid according to a weight ratio of 1:(1-6). The hydroxychloroquine sulfate and polyglutamic acid polymer is applied to treatment of discoid lupus erythematosus and systemic lupus erythematosus. The hydroxychloroquine sulfate and the polyglutamic acid are cross-linked to obtain the hydroxychloroquine sulfate and polyglutamic acid polymer, and hydroxyls of the hydroxychloroquine sulfate are modified to change certain original physiochemical properties thereof, improve pharmaceutical activity, enhance curative effects, reduce side effects, improve the sustained release effects of the drug and reduce the medication frequency of a patient; when the hydroxychloroquine sulfate and polyglutamic acid polymer is used for treating discoid lupus erythematosus and systemic lupus erythematosus, the drug can reach a destination lesion location under protection of the polyglutamic acid in a slow degradation process, so that the aim of accurate treatment is fulfilled, and the hydroxychloroquine sulfate and polyglutamic acid polymer has a certain clinical application value.
Owner:陕西省生物农业研究所
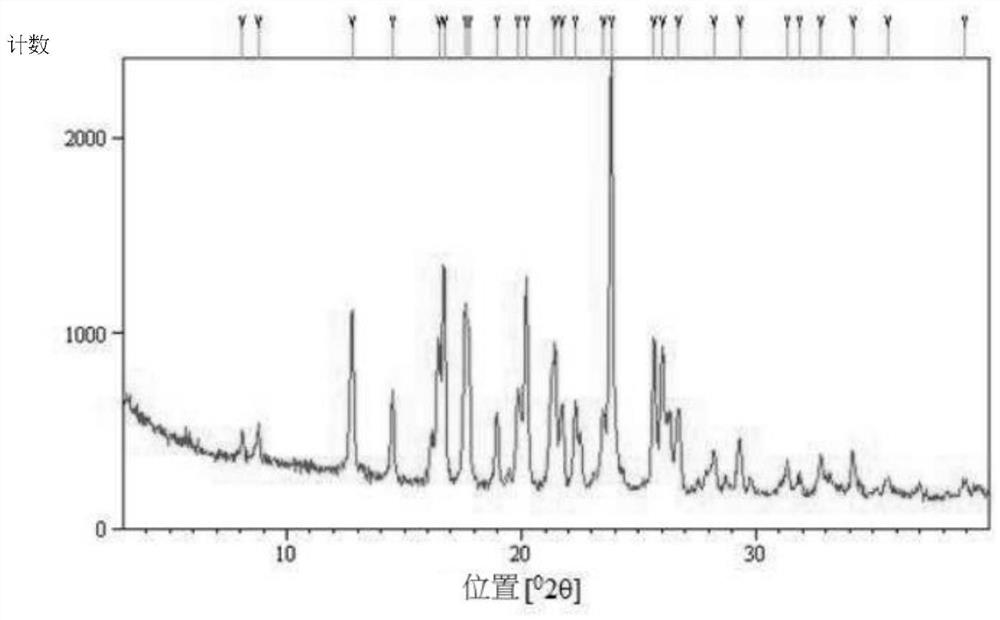
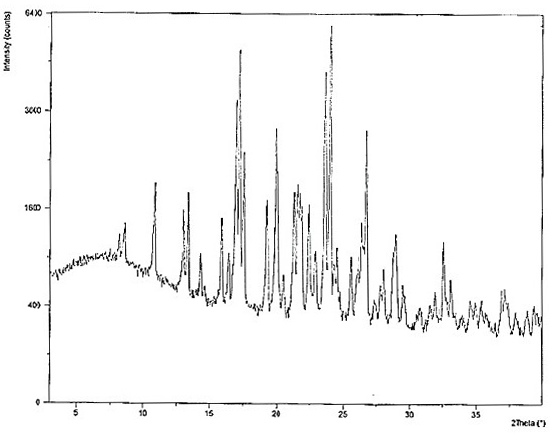
Hydroxychloroquine sulfate crystal form B and preparation method thereof
InactiveCN112480000AEasy to prepareLow hygroscopicityOrganic active ingredientsOrganic chemistry methodsPharmaceutical drugCombinatorial chemistry
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a hydroxychloroquine sulfate crystal form B and a preparation method thereof, and XRD and DSC are usedfor characterization. The hydroxychloroquine sulfate crystal form B provided by the invention is simple in preparation method, low in hygroscopicity, good in stability, capable of forming a regular crystal form and higher in solubility, thereby being beneficial to process treatment of medicines, improvement of physical and chemical properties and improvement of patent medicine properties.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
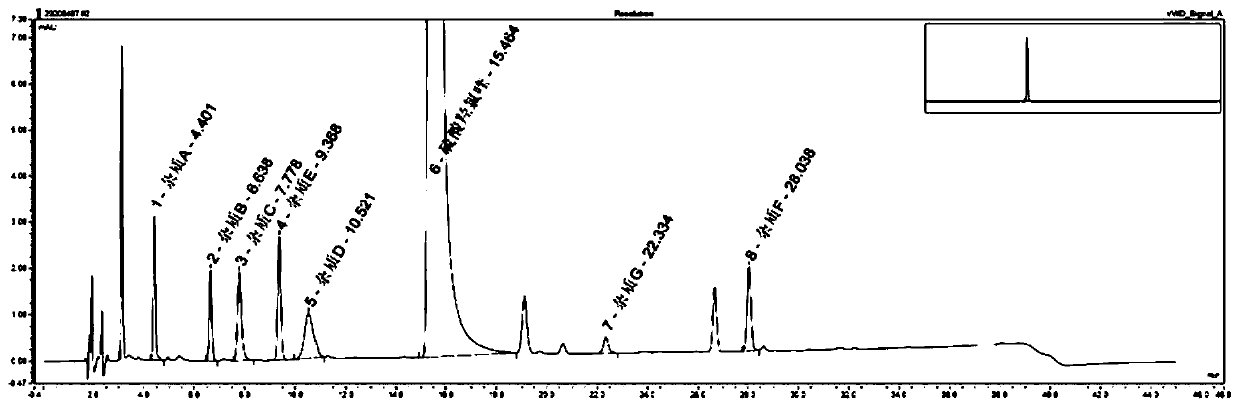
Method for detecting hydroxychloroquine sulfate related substances and application thereof
ActiveCN111551645AEasy to separateHigh sensitivityComponent separationHydroxychloroquine SulfateSolvent
The invention discloses a method for detecting hydroxychloroquine sulfate related substances. The method comprises the following step: carrying out separation and detection by adopting reverse liquidchromatography, wherein chromatographic conditions are as follows: a mobile phase is a mixed solvent consisting of a water-soluble organic solvent and a buffer solution; wherein the buffer solution isan ammonium acetate solution or an ammonium formate solution; wherein the pH value of the buffer solution is 9.0 to 10.5. The detection method disclosed by the invention can be used for simultaneously detecting seven impurities recorded in quality standards of hydroxychloroquine sulfate raw materials in European Pharmacopoeia 9.8 versions in hydroxychloroquine sulfate raw materials and preparations, and has the advantages of good separation effect, high sensitivity, no blank interference, low detection cost, high analysis speed and the like.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
Method for prevention and treatment of a viral-mediated infectious disease
A method for prevention or treatment of a viral-mediated infectious disease in a mammal. The mammal may be a human being. A therapeutic dose of a composition is administered, via an inhalation delivery apparatus, to the mammal. The composition includes microparticles and / or nanoparticles. The microparticles and / or nanoparticles include a first pharmaceutically active agent and a second pharmaceutically active agent. The first pharmaceutically active agent includes unfractionated heparin (UFH), Low Molecular Weight Heparin (LMWH), sulfated non-anticoagulant heparin (S-NACH) or combinations thereof. The second pharmaceutically active agent includes 10-30 mg of hydroxychloroquine in a form of hydroxychloroquine sulfate, 10-30 mg of favipiravir, or a combination thereof. The viral-mediated infectious disease is caused by one or more viruses in the mammal.
Owner:VIROTHERA PHARM LLC
Hydroxychloroquine sulfate and preparation method thereof
PendingCN113185459AGood pollution effectSpeed up the reaction processOrganic chemistryHydroxychloroquinePtru catalyst
The invention discloses hydroxychloroquine sulfate and a preparation method thereof, and relates to the technical field of medicinal chemistry. The preparation method comprises the steps of mixing 4, 7-dichloroquinoline with a hydroxychloroquine side chain, carrying out heating condensation in the presence of an organic base catalyst, adding water and liquid, and carrying out cooling crystallization to obtain hydroxychloroquine; and dissolving hydroxychloroquine in an ethyl acetate and ethanol aqueous solution, heating, dissolving and clarifying, dropwise adding concentrated sulfuric acid, cooling, crystallizing, filtering and drying to obtain hydroxychloroquine sulfate. The method has the beneficial effects that a solvent-free reaction is used, and a catalyst is added, so that high pollution is avoided, the reaction process is accelerated, and the operation is simple; and meanwhile, the HPLC purity of the obtained hydroxychloroquine refined product is not less than 96.50%, the maximum single impurity content is less than 0.10%, the yield can reach 85%, the HPLC purity of hydroxychloroquine sulfate is not less than 98.00%, the maximum single impurity content is less than 0.10%, and the yield can reach 90%.
Owner:JIANGXI GUOYAO PHARMA LLC +1
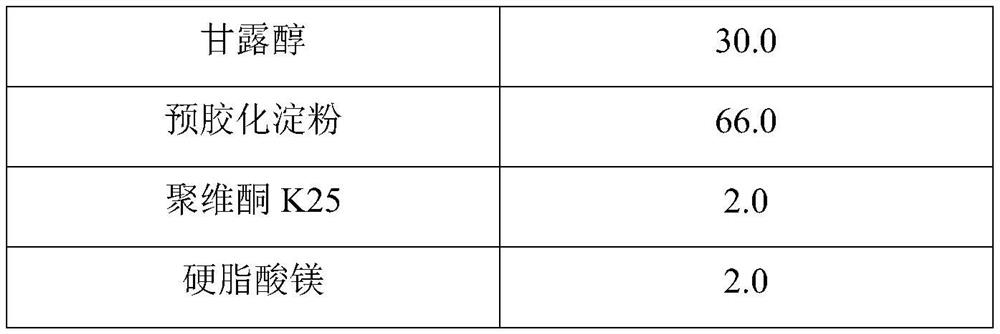
Hydroxychloroquine sulfate pharmaceutical preparation
PendingCN113244180ASolve the problem of easy stickingSolve the problem of poor compressibility caused by high friabilityOrganic active ingredientsAntipyreticDrugs preparationsHydroxychloroquine Sulfate
The invention provides a hydroxychloroquine sulfate pharmaceutical preparation. The hydroxychloroquine sulfate pharmaceutical preparation comprises the following components in percentage by weight: 50-75% of hydroxychloroquine sulfate, 5-15% of mannitol, 15-35% of pregelatinized starch, 0.5-2.0% of a water-soluble adhesive and 0.5-2.0% of a lubricant. According to the hydroxychloroquine pharmaceutical preparation disclosed by the invention, the mannitol and the pregelatinized starch are used as filling agents, and are matched with the adhesive and the lubricant, so that the problem that the hydroxychloroquine pharmaceutical preparation is extremely likely to stick and the problem of poor compressibility caused by high friability of tablets can be solved, the hydroxychloroquine pharmaceutical preparation has good dissolution property, and the dissolution effect is consistent with that of a reference reagent.
Owner:BEIJING WINSUNNY PHARMA CO LTD
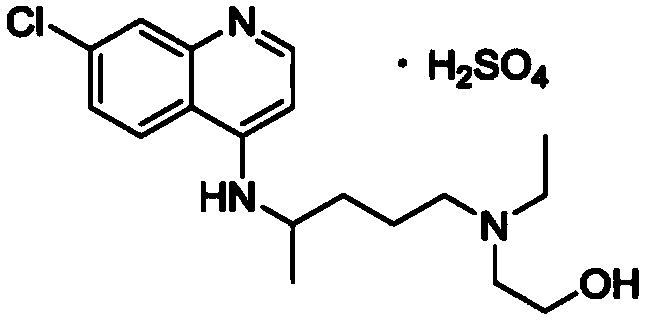
Hydroxychloroquine sulfate as well as crystal form and preparation method of enantiomer of hydroxychloroquine sulfate
PendingCN111793026AGood chemical stabilityReduce riskOrganic compound preparationOrganic chemistry methodsNitrogen oxidesEnantiomer
The invention relates to hydroxychloroquine sulfate as well as a crystal form and a preparation method of an enantiomer of hydroxychloroquine sulfate. The invention provides a crystal form A hydroxychloroquine sulfate. An X-ray powder diffraction pattern of the crystal form A hydroxychloroquine sulfate has characteristic peaks at 10.8 degrees, 13.0 degrees, 13.3 degrees, 16.9 degrees, 17.2 degrees, 17.5 degrees, 19.9 degrees, 21.3 degrees, 23.5 degrees, 24.0 degrees and 26.7 degrees + / -0.2 degrees, and does not contain hydroxychloroquine nitrogen oxide; the invention provides a hydroxychloroquine crystal, the X-ray powder diffraction pattern has characteristic peaks at 7.5 degrees, 14.9 degrees, 16.5 degrees, 19.2 degrees, 19.6 degrees, 22.8 degrees, 23.6 degrees and 26.7 degrees+ / -0.2 degree; the invention provides an S-hydroxychloroquine sulfate monohydrate, the X-ray powder diffraction pattern has characteristic peaks at 12.2 degrees, 13.0 degrees, 14.9 degrees, 17.8 degrees, 22.7 degrees, 23.3 degrees, 25.0 degrees and 26.2 degrees + / -0.2 degrees; the invention also provides R-hydroxychloroquine sulfate, the X-ray powder diffraction pattern has characteristic peaks at 12.2 degrees, 13.0 degrees, 14.9 degrees, 17.8 degrees, 22.7 degrees, 23.3 degrees, 25.0 degrees and 26.2 degrees + / -0.2 degrees.
Owner:珠海润都制药股份有限公司
Preparation method of hydroxychloroquine sulfate
PendingCN111423373ASimple processGood reproducibilityOrganic chemistryHydroxychloroquineReaction temperature
The invention relates to a preparation method of hydroxychloroquine sulfate, and belongs to the technical field of medicine synthesis. According to the preparation method of hydroxychloroquine sulfate, 7-chloro-4-fluoroquinoline and 5-(N-ethyl-N-2-hydroxyethyl amine)-2-pentylamine are subjected to a reaction to prepare hydroxychloroquine, and then hydroxychloroquine and sulfuric acid are salifiedto prepare hydroxychloroquine sulfate. The 7-chloro-4-fluoroquinoline is prepared from 4,7-dichloroquinoline through a halogen exchange reaction. The preparation method of hydroxychloroquine sulfate has the advantages of low reaction temperature, short reaction time, fewer byproducts, simple technique and favorable reproducibility, and is beneficial to industrial production.
Owner:REYOUNG PHARMA
Combined medicine containing artemisinin
ActiveCN105326840ASmall toxicityDelay drug resistanceOrganic active ingredientsAntiparasitic agentsAdjuvantHydroxychloroquine Sulfate
The invention provides a combined medicine. The combined medicine consists of artemisinin which serves as a first active ingredient, hydroxychloroquine sulfate which serves as a second active ingredient and optional pharmaceutically acceptable adjuvants. The combined medicine can be used for treating malaria more effectively, and the combined medicine can relieve drug toxicity and delay the generation of drug resistance.
Owner:INST OF SCI & TECH GUANGDONG UNIV OF CHINESE MEDICINE
Purification method of hydroxychloroquine sulfate
InactiveCN111377861AImprove the purification effectSimple and safe operationOrganic chemistryHydroxychloroquine SulfateSolvent
The invention discloses a purification method of hydroxychloroquine sulfate, which comprises the following steps: (1) dissolving a hydroxychloroquine sulfate crude product in water or a mixed solventof water and a first solvent to obtain a mixed solution I; (2) adding a second solvent into the mixed solution I, and carrying out reflux crystallization; and (3) cooling the reaction product, and filtering and drying the reaction product to obtain a fine hydroxychloroquine sulfate product. The method is excellent in purification effect, so that hydroxychloroquine sulfate with the purity of 99.5%or above and the particle size Dx (90) larger than or equal to 200 microns is prepared. The method is easy and convenient to operate, low in cost, safe, environmentally friendly and suitable for large-scale production.
Owner:CINKATE PHARMA INTERMEDIATES +1
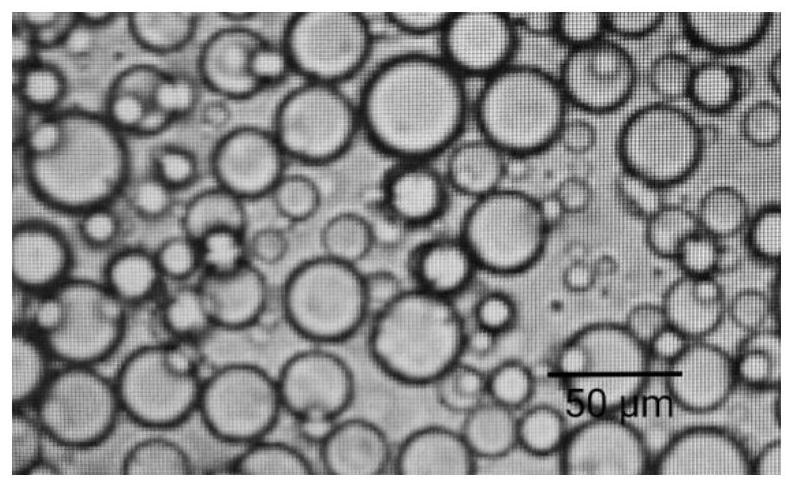
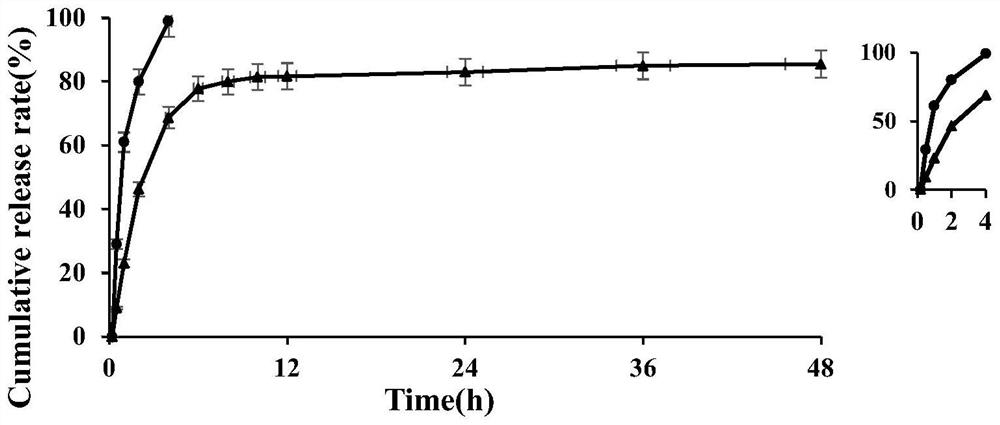
Hydroxychloroquine sulfate sustained release microsphere for articular cavity injection and preparation method thereof
ActiveCN114796126AFlat shapeUniform particle size distributionOrganic active ingredientsAntipyreticHydroxychloroquineMicrosphere
The invention discloses a hydroxychloroquine sulfate sustained-release microsphere for articular cavity injection and a preparation method thereof. The preparation method comprises the following steps: dissolving a surfactant in an organic solvent to form an oil phase; adding hydroxychloroquine sulfate and gelatin into water to obtain a water phase; dropwise adding the water phase into the oil phase, stirring and emulsifying to form a W / O type emulsion; transferring the W / O emulsion into an ice bath, and adding a curing agent for cross-linking curing; and then adding isopropanol for dehydration, suction filtration, washing and drying to obtain the hydroxychloroquine sulfate sustained-release microspheres for articular cavity injection. The prepared hydroxychloroquine microspheres can stably and continuously release drugs for more than 48 hours, a sustained-release preparation is obtained, the administration frequency can be reduced, the total dosage can be reduced, the compliance of patients can be improved, and the clinical application potential is good.
Owner:复旦大学附属中山医院青浦分院(上海市青浦区中心医院)
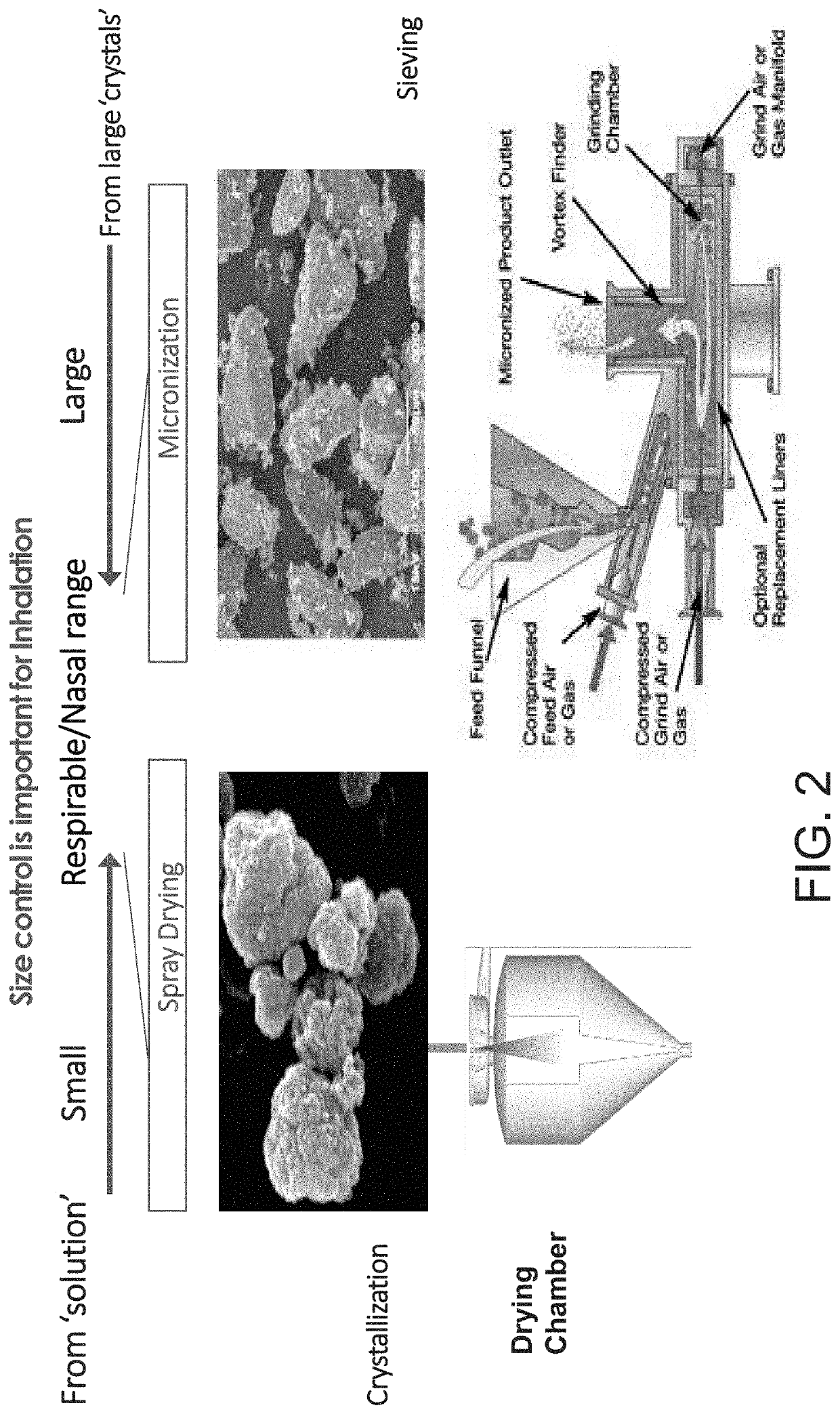
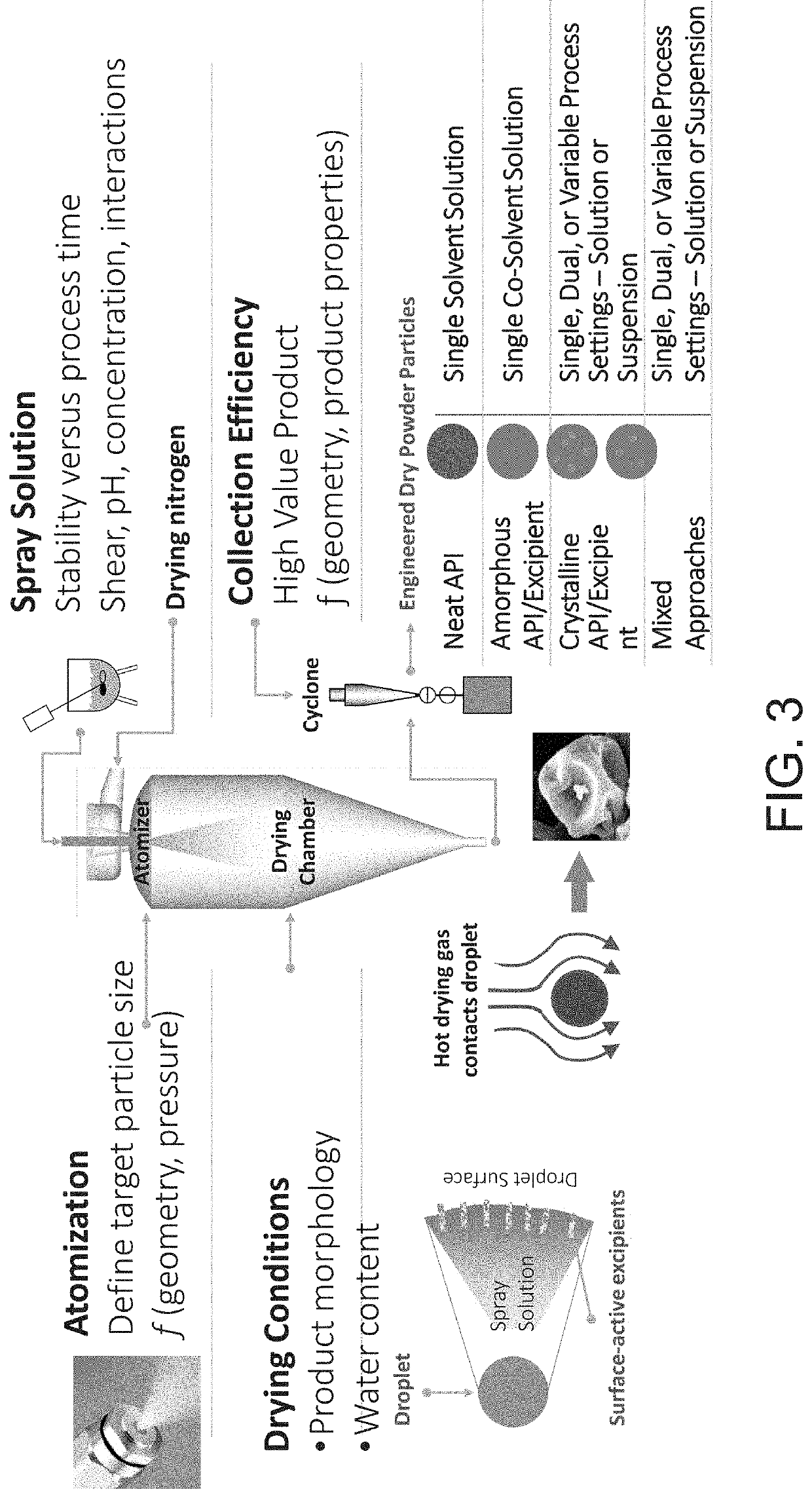
Inhalant containing chloroquine therapeutic agent and preparation method of inhalant
InactiveCN113559086AImprove stabilityImprove the protective effectOrganic active ingredientsPowder deliveryOral medicationInhalation
The invention provides a chloroquine inhalant and a preparation method thereof, and belongs to the field of medicine preparations. The inhalant comprises chloroquine phosphate or hydroxychloroquine sulfate, a stabilizer and a diluent, can improve use efficiency, has a preventive effect, such as a new epidemic situation-COVID-19, and can take effect quickly. The powder inhalation has better stability, has the effects of masking taste, enhancing stability and protecting organism biological membranes on chloroquine phosphate or hydroxychloroquine sulfate after being wrapped by the stabilizer, is absorbed through the lungs, has a high targeting effect, also can effectively avoid the first-pass effect caused by the liver during oral administration, and improves bioavailability.
Owner:DISCOVERY SHENZHEN NEW MEDICINES DEV CO LTD
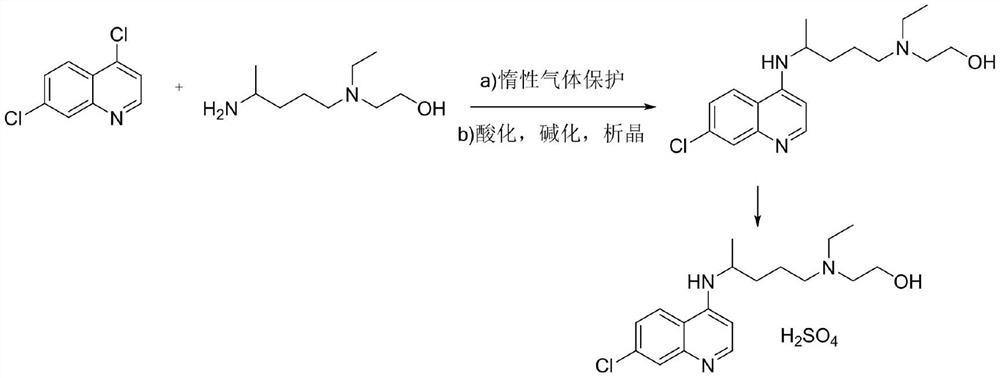
A kind of preparation method of sulfasalazine intermediate
ActiveCN110862345BLow process temperatureReduce manufacturing costOrganic chemistryPtru catalystSide chain
The present invention provides a kind of preparation method of sulfasalazine intermediate, and its preparation method comprises the following steps: Step 1: add 4-amino-7-chloroquinoline in the mixing container that organic solvent is housed, add to described mixing container Inert gas is introduced into the medium, and the temperature is raised to 65-75°C; Step 2: After stirring for a certain period of time, add side chains and catalysts to the mixing container, continue to heat up to 85±3°C, and continue to stir; Step 3: Wait for the reaction After the end, cool down to 10±2°C and stand for separation to obtain the hydroxychloroquine matrix; Step 4: add sulfate solution to the hydroxychloroquine matrix, stir for 3.5-5 hours, and separate the solids in the solution, and further bake Dry access to hydroxychloroquine sulfate material. The preparation method of the sulfasalazine intermediate of the present invention has a simple preparation process and is suitable for popularization and use.
Owner:SUZHOU HUANGHE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com