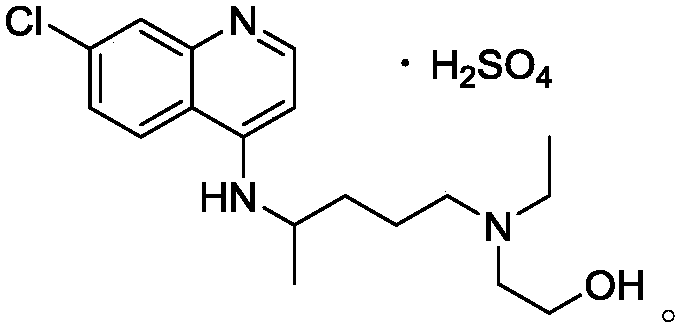
Hydroxychloroquine sulfate crystal form A and preparation method thereof
A technology of hydroxychloroquine sulfate crystal and hydroxychloroquine sulfate, which is applied in the field of hydroxychloroquine sulfate crystal form A and its preparation, can solve the problems of poor stability of hydroxychloroquine sulfate, and achieve the effects of easy industrial production, good stability, and stable and reliable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis of embodiment 1 hydroxychloroquine
[0040] Add 100g of 4,7-dichloroquinoline and 130g of 5-(N-ethyl-N-2-hydroxyethylamine)-2-pentylamine into the reactor, pass through argon protection, raise the temperature to 70°C to make 4 , Dissolve 7-dichloroquinoline, heat up to 115°C for 10 minutes, heat up to 137°C for 10 hours, cool down (below 80°C) after the reaction is complete, and adjust pH>12 with sodium hydroxide solution (mass concentration: 6%), Extracted with dichloromethane, washed with water until neutral, and distilled off the dichloromethane under reduced pressure to obtain 157 g of crude hydroxychloroquine with a yield of 92.5%. Add 200g of acetone and 250g of methyl acetate to the crude product of hydroxychloroquine, heat to dissolve at 65°C, slowly cool down to 10°C over 4 hours, filter, and wash the filter cake with a mixed solution of acetone and methyl acetate to obtain a wet product of hydroxychloroquine. Dry at 60°C for 4 hours to obtain dr...
Embodiment 2-19
[0041] The preparation of embodiment 2-19 hydroxychloroquine sulfate crystal form A
[0042] Dissolve the hydroxychloroquine obtained in Example 1 in an organic solvent, add dropwise an aqueous solution of sulfuric acid with a mass fraction of 40-60% until cloudy, after the dropwise addition, heat up to 35°C-55°C, keep warm for crystallization, and then lower the temperature by 15°C Then keep it warm for 1h, filter, and dry at 50°C to obtain hydroxychloroquine sulfate crystal form A.
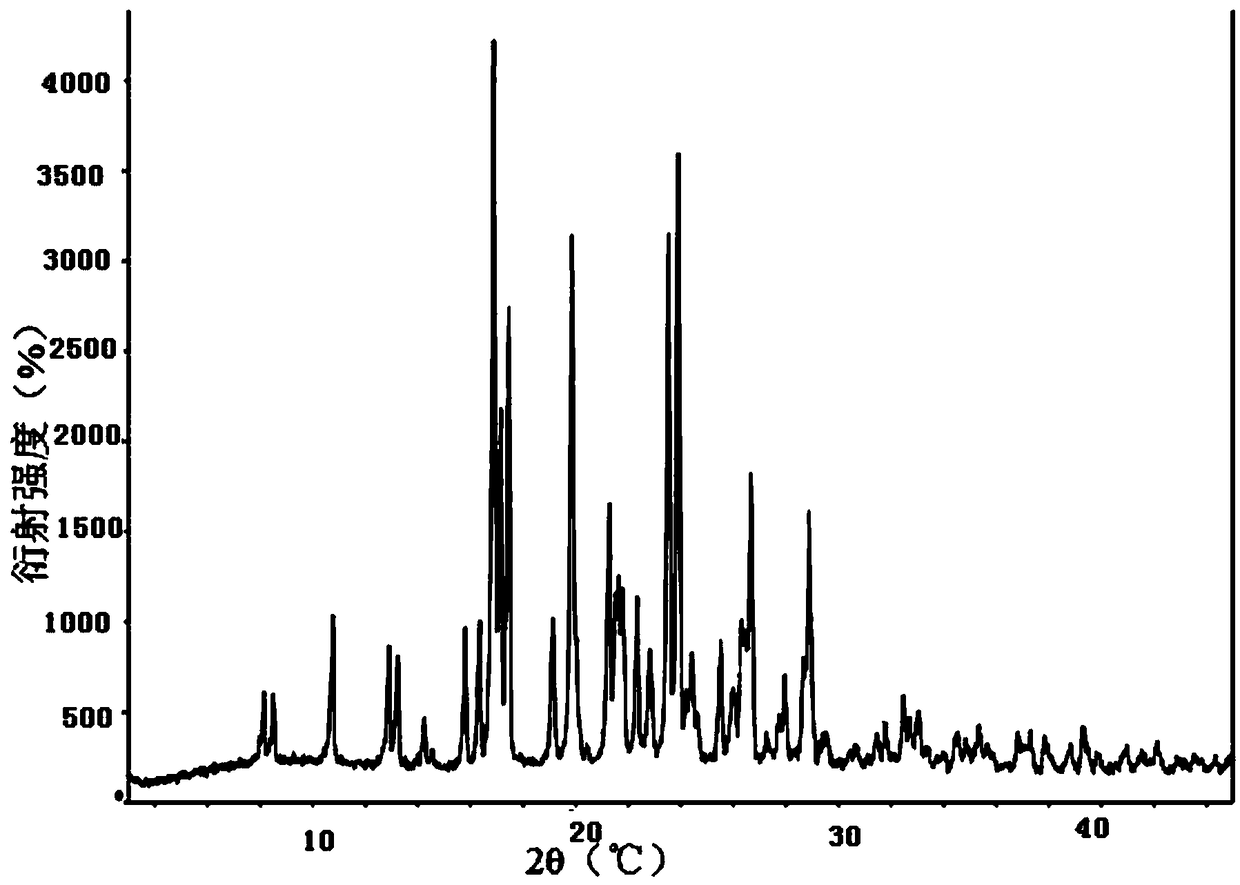
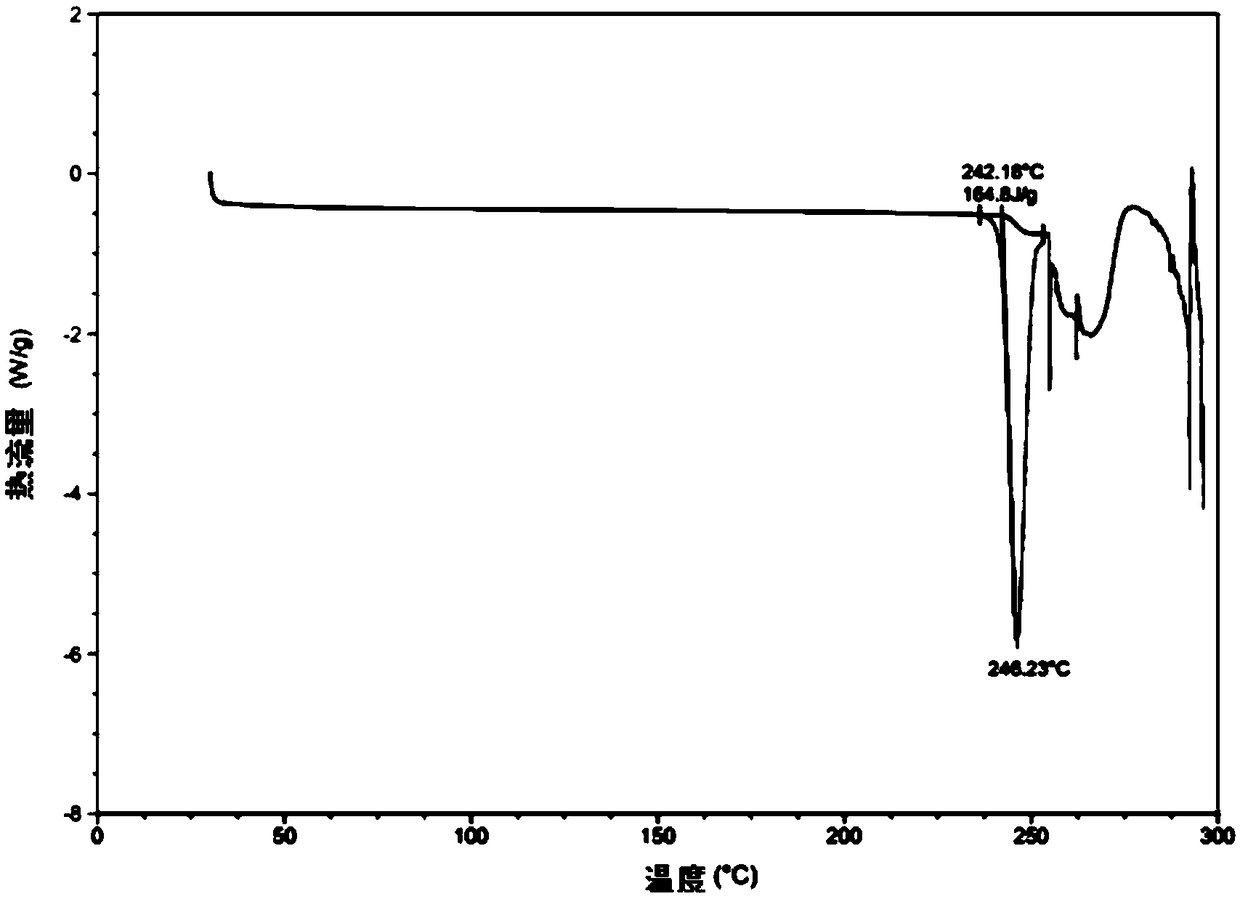
[0043] Wherein, the hydroxychloroquine sulfate crystal form A prepared in Example 2 has the following properties: figure 1 The X-ray powder diffraction spectrum shown has characteristic peaks at 2θ=16.9°, 17.1°, 17.5°, 19.9°, 21.3°, 23.5°, 23.9°, and 26.7°; its DSC spectrum is as figure 2 As shown, there is an endothermic peak at 246 °C.
[0044] The concrete condition of table 1 embodiment 2-19
[0045]
[0046]
[0047]
[0048] In the above table, the heat preservation in the heat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com