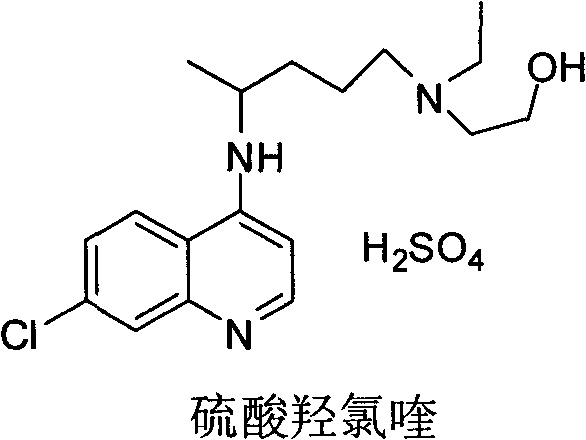
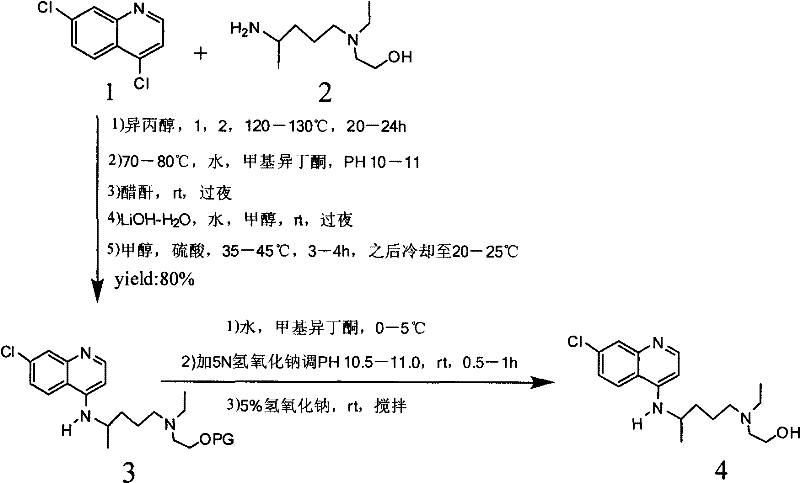
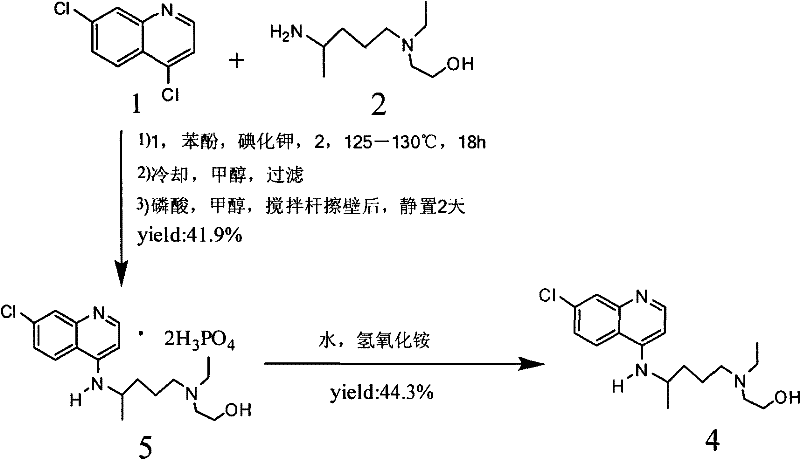
Industrial preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine, which is applied in the field of chemistry or pharmaceutical chemistry, can solve problems such as complicated steps, high impurity content in products, and increased post-processing pressure, so as to reduce production costs and improve product yield and quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of hydroxychloroquine sulfate
[0043] a, the preparation of hydroxychloroquine
[0044] Calculate the amount of raw materials according to the theoretical production amount of 153g of hydroxychloroquine; in a 1000mL three-necked round-bottomed flask, add 90g of 4,7-dichloroquinoline and 141g of isopropanol, heat up to a temperature of 60°C under stirring conditions to completely dissolve, slowly Add 95.13g of hydroxyquine side chain dropwise, start timing after the dropwise addition, and gradually heat up to 120-125°C after 7 hours, then keep it at 120°C-125°C for 16 hours until the high performance liquid phase detection reaches the end of the reaction, and wait for the reaction After completion, cool the reaction solution to a temperature of 50-60°C, add 6% sodium hydroxide solution, and make the pH value >12 after alkalization, while continuing to cool down to 20-25°C. After the temperature was reached, 279g of dichloromethane was adde...
Embodiment 2
[0048] Embodiment 2, the preparation of hydroxychloroquine sulfate
[0049] a, the preparation of hydroxychloroquine
[0050] Calculate the amount of raw materials according to the theoretical production amount of 153g of hydroxychloroquine; in a 1000mL three-necked round-bottomed flask, add 90g of 4,7-dichloroquinoline and 141g of isopropanol, heat up to a temperature of 60°C under stirring conditions to completely dissolve, slowly Add 95.13g of hydroxyquine side chain dropwise, start timing after the dropwise addition, and gradually heat up to 120-125°C after 10 hours, then keep it at 120°C-125°C for 16 hours until the end of the reaction is reached by HPLC detection, and wait for the reaction After completion, cool the reaction solution to a temperature of 50-60°C, add 6% sodium hydroxide solution, and make the pH value >12 after alkalization, while continuing to cool down to 20-25°C. After the temperature was reached, 279g of dichloromethane was added for the first time, ...
Embodiment 3
[0054] Embodiment 3, the preparation of hydroxychloroquine sulfate
[0055] a, the preparation of hydroxychloroquine
[0056] Calculate the amount of raw materials according to the theoretical production amount of 153g of hydroxychloroquine; in a 1000mL three-necked round-bottomed flask, add 90g of 4,7-dichloroquinoline and 141g of isopropanol, heat up to a temperature of 60°C under stirring conditions to completely dissolve, slowly Add 95.13g of hydroxyquine side chain dropwise, start timing after the dropwise addition, and gradually heat up to 120-125°C after 12 hours, then keep it at 120°C-125°C and keep it warm for 16 hours until the high-performance liquid phase detection reaches the end of the reaction, and wait for the reaction After completion, cool the reaction solution to a temperature of 50-60°C, add 6% sodium hydroxide solution, and make the pH value >12 after alkalization, while continuing to cool down to 20-25°C. After the temperature was reached, 279g of dichlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com