Crystals of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and crystals, which is applied in the field of crystals of hydroxychloroquine sulfate, and can solve problems such as visual impairment and retinal damage in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
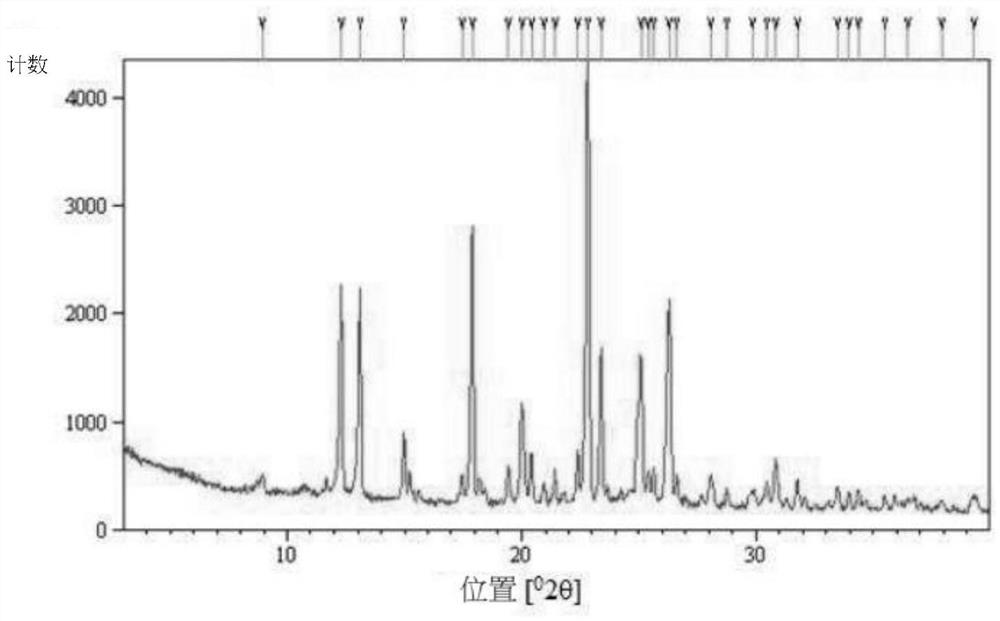
[0041] Example 1: Preparation and Characterization of Type A Crystals of (S)-(+)-Hydroxychloroquine Sulfate
[0042] Form A crystals of (S)-(+)-hydroxychloroquine sulfate were prepared according to the method described below.
[0043] Preparation of (+)-hydroxychloroquine solution in salt-free form
[0044]Dissolve 5 g of (±)-hydroxychloroquine sulfate in 11 mL of water at room temperature. Then, 25 mL of ethyl acetate and 14 mL of aqueous sodium hydroxide solution (2M) were added to the resulting solution, and the reaction solution was stirred at room temperature for 1 hr. The organic phase was separated from the aqueous phase, dried over sodium sulfate and concentrated to an oil. This oil was dissolved in 5 mL of acetonitrile. 45 mL of water was added dropwise to the solution at room temperature. The resulting solution was stirred overnight at room temperature and a white precipitate formed. The precipitate was isolated by centrifugation and dried under vacuum at room t...
example 2
[0087] Example 2: Preparation and Characterization of Type B Crystals of (S)-(+)-Hydroxychloroquine Sulfate
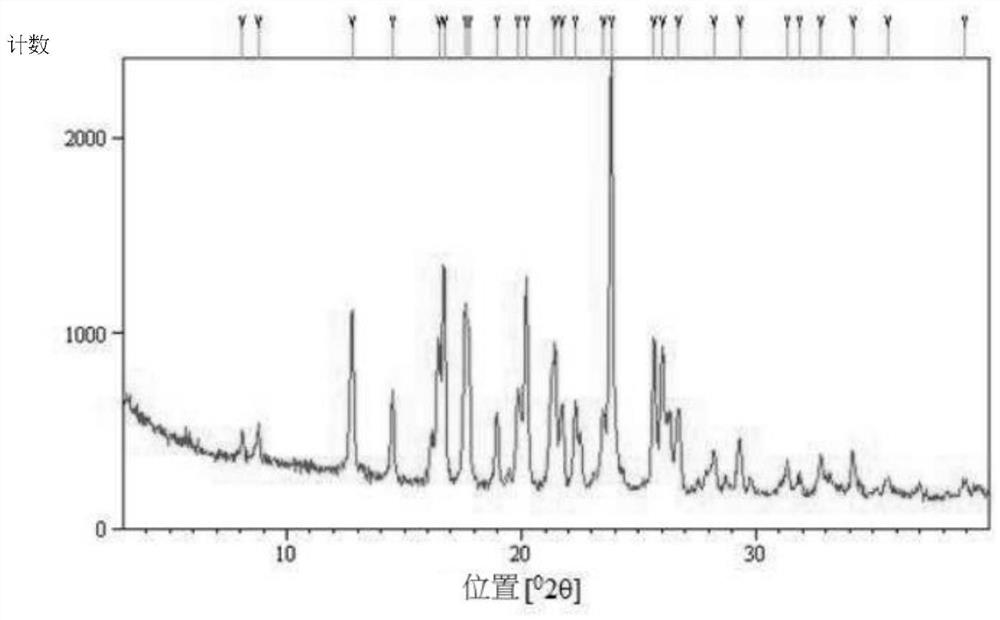
[0088] To prepare type B crystals of (S)-(+)-hydroxychloroquine sulfate, type A crystals (1.5 g) of (S)-(+)-hydroxychloroquine sulfate were dissolved in ethanol (5 mL), and then dissolved at 50 Stirred at °C for 4 hours to precipitate and obtain (S)-(+)-hydroxychloroquine sulfate type B crystals (1.5 g), ee 99%.
[0089] Form B crystals were analyzed by PXRD. Such as image 3 As shown, it is 8.1±0.1°, 8.8±0.1°, 12.8±0.1°, 14.5±0.1°, 16.7±0.1°, 17.6±0.1°, 19.0±0.1°, 20.2±0.1°, The diffraction peaks at 21.4±0.1°, 21.7±0.1°, 22.3±0.1°, 23.8±0.1°, 25.7±0.1°, 26.0±0.1°, 26.7±0.1°, 28.2±0.1° and 29.3±0.1° are feature.
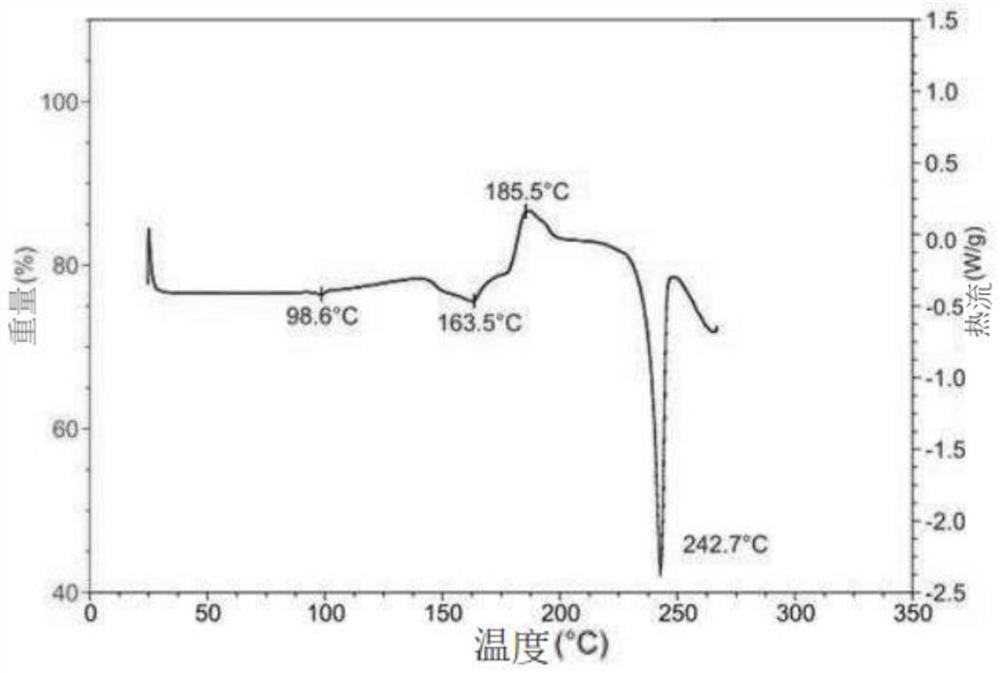
[0090]Crystals were also analyzed by DSC. Such as Figure 4 As shown, it has an endothermic peak at 245.2±0.1°C in the differential scanning calorimetry curve.
example 3
[0091] Example 3: Hygroscopicity of (S)-(+)-type A crystal, B-type crystal and amorphous form of hydroxychloroquine sulfate
[0092] Use the VTI-SA vapor adsorption analyzer of TAInstruments (New Castle, DE, USA) to carry out dynamic vapor adsorption analysis on (S)-(+)-hydroxychloroquine sulfate A-type crystal, B-type crystal and amorphous form, to compare its hygroscopicity. Each was exposed to a series of steps in relative humidity (RH) change at 25°C, i.e., each step increased 5% RH from 0 to 95% RH, and each subsequent step decreased 5% RH from 95% to 5% RH. Then record the moisture content of the humidity increase step and the humidity decrease step, respectively generate as Figure 7 , Figure 8 and Figure 9 The adsorption and desorption curves are shown.
[0093] Referring to the adsorption curves in the figure, (i) Type A crystals do not adsorb water at RHs up to 85%, and Type B crystals only adsorb water at RHs higher than 60%, compared to amorphous forms at RH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com