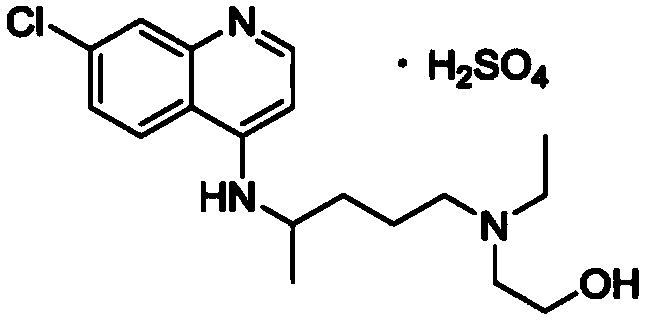
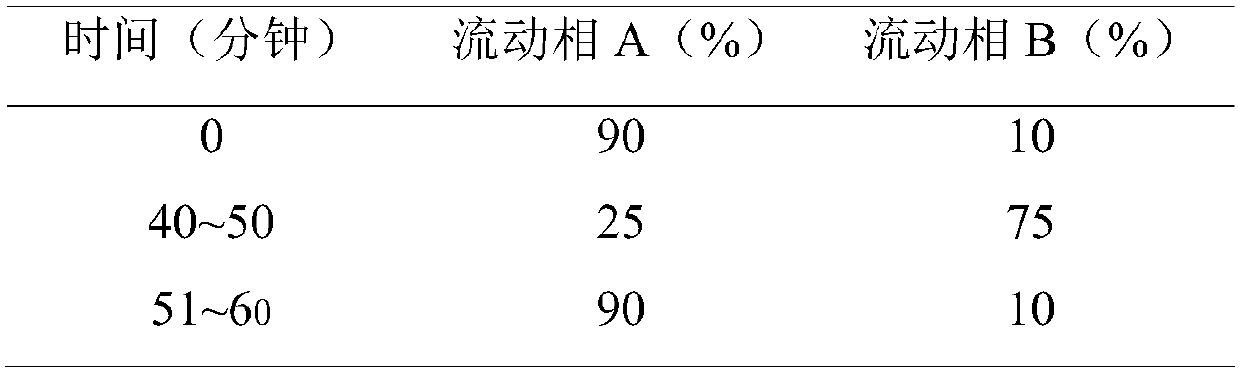
Purification method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and purification method, which is applied in the field of purification of hydroxychloroquine sulfate, and can solve the problems of no suitable method for finished product control, clarity, acidity and unqualified crystal form, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Preparation of crude hydroxychloroquine sulfate:
[0070] Put 0.91Kg of 4,7-dichloroquinoline, 0.9kg of phenol and 10g of potassium iodide into a 20L reactor, stir and heat to 120°C, and slowly add 5-(N-ethyl-N-2-hydroxyethylamino)-2 - Pentylamine 1.32Kg, after dropping, continue stirring at 125-130°C for 18 hours. Cool to room temperature, add 20% sodium hydroxide lye to adjust to pH 12, extract with toluene three times, concentrate under reduced pressure to remove toluene, dissolve the residue in 1.5L absolute alcohol, cool to room temperature, slowly add concentrated sulfuric acid, adjust pH to 6-7.5, stirring, crystallization slowly. Store at 5°C for more than 6 hours. Filter to obtain the wet product of hydroxychloroquine sulfate crude product. Vacuum dried at 80°C for 4 hours. Obtained 0.89kg crude product of hydroxychloroquine sulfate, purity 95%, mp235-240 ℃, yield 45.76%.
Embodiment 2
[0072] Add 600g of crude hydroxychloroquine sulfate, 600ml of water and 1200ml of ethanol into a 10L round-bottomed three-neck flask respectively, then stir and heat to 70°C, continue to insulate and stir until completely dissolved, slowly add 6L of ethanol, then heat to reflux (78-80 ℃), refluxing and crystallizing for 1 hour, then stirring to lower the temperature to 30-40 ℃, continuing to stir for 1 hour, filtering, rinsing with a small amount of ethanol, and drying the filter cake to obtain 578.5 g of white solid with a yield of 96.41%. High performance liquid chromatography purity 99.85%, the largest single impurity 0.05%, particle size D x (90) is 339 μm. The main characteristic peaks measured by the 2θ reflection angle in the X-ray powder diffraction pattern: 12.95°, 17.10°, 17.21°, 17.47°, 19.90°, 21.15°, 23.5±0.2°, 23.9±0.2°, 26.7±0.2°.
Embodiment 3
[0074] Add 80g of crude hydroxychloroquine sulfate, 40ml of water and 120ml of ethanol into a 1L round-bottomed three-necked flask, then stir and heat to reflux, continue to reflux for 0.5h, slowly add 640ml of ethanol, stir and crystallize under reflux for 0.5, then stir and cool down to 40°C , continue to stir for 3 hours, filter, rinse with a small amount of ethanol, and dry the filter cake to obtain 70.1 g of white solid, with a yield of 87.67%. The purity by high performance liquid chromatography is 99.58%, the maximum single impurity is 0.09%, and the particle size Dx(90) is 259 μm. The main characteristic peaks determined by the 2θ reflection angle in the X-ray powder diffraction pattern: 12.87°, 16.85°, 17.11°, 17.45°, 19.84°, 21.25°, 23.52°, 23.90°, 26.64°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com