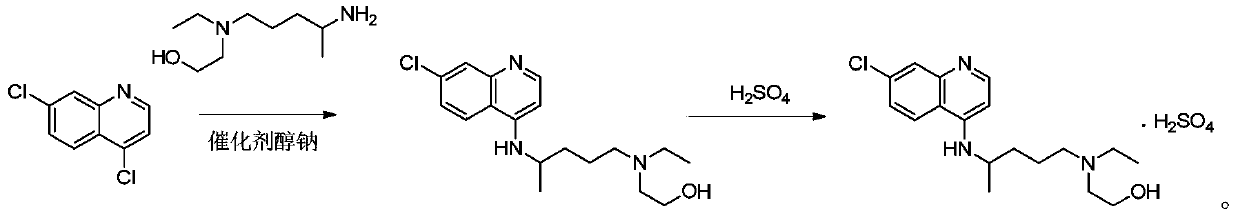
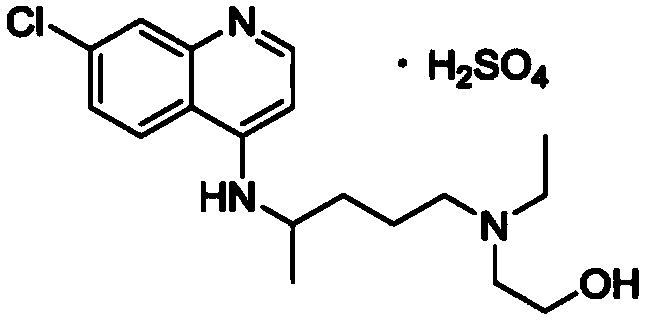
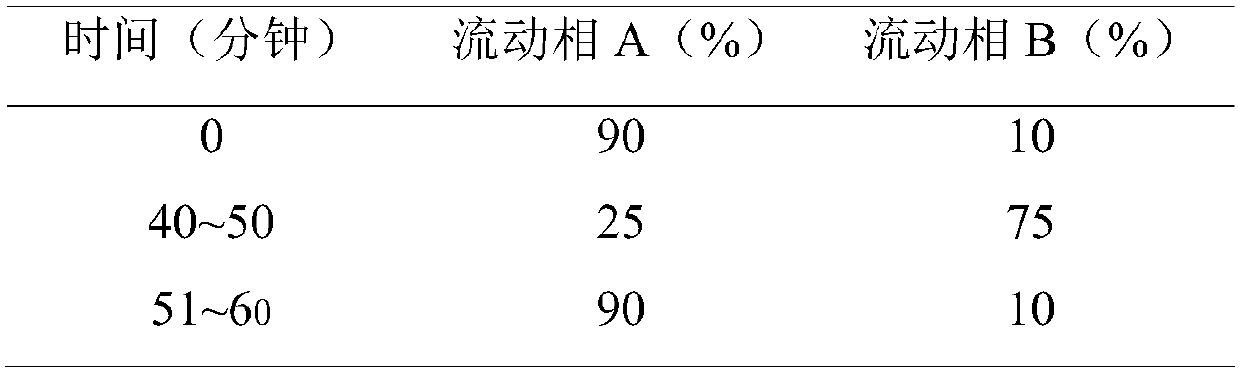
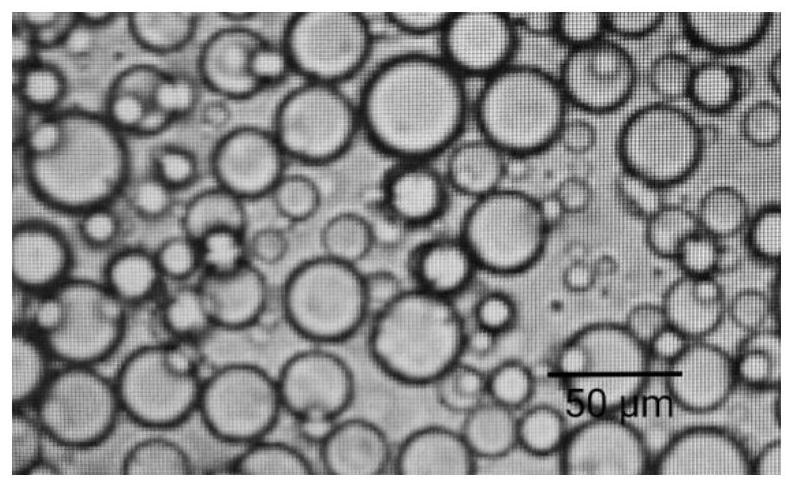
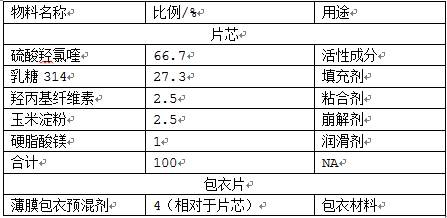
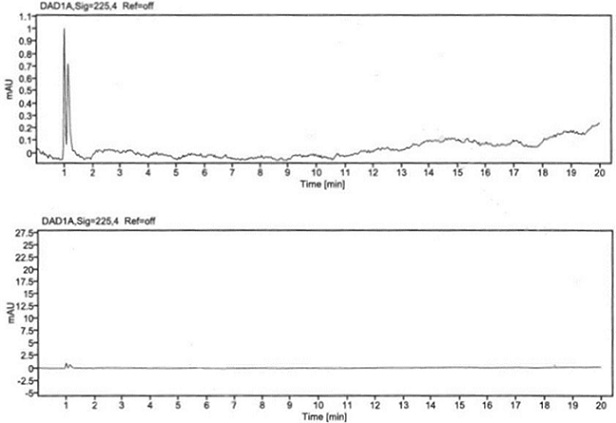
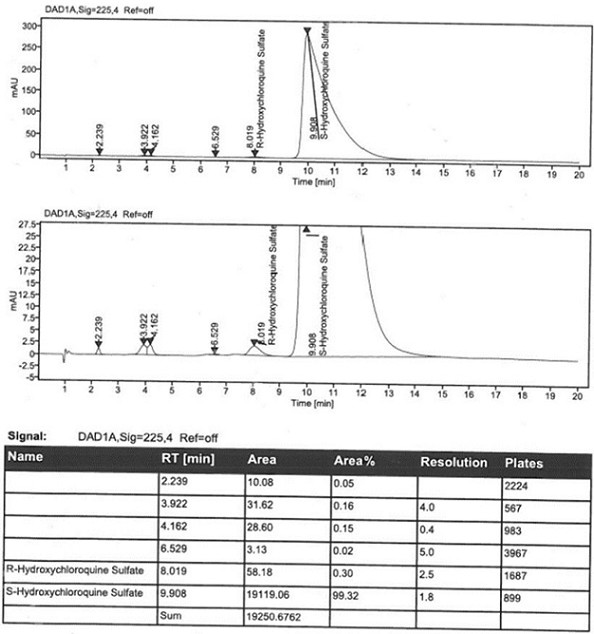
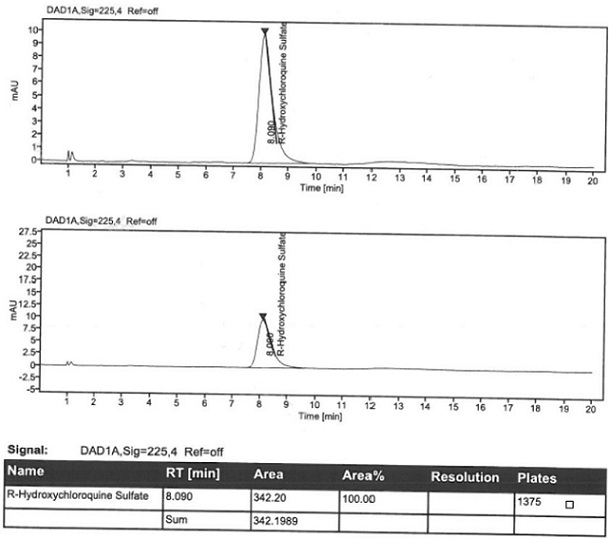
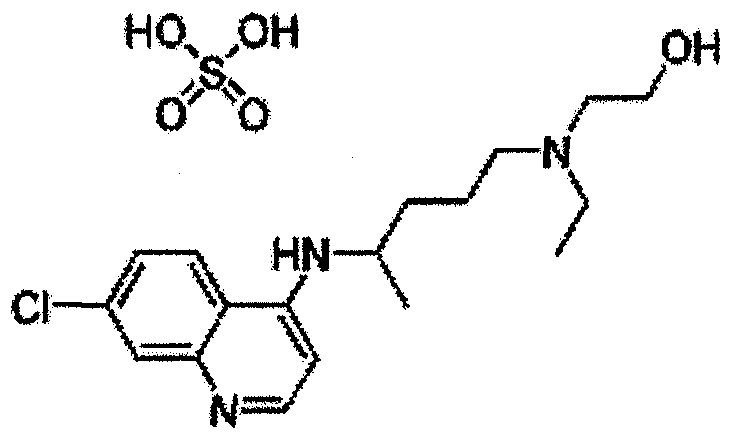
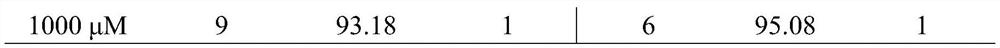Patents
Literature
30 results about "Hydroxychloroquine sulphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
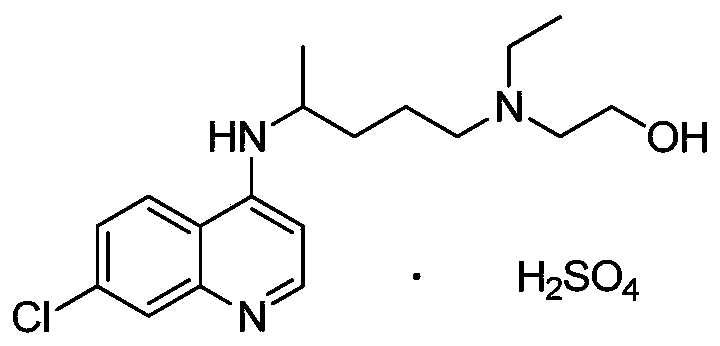
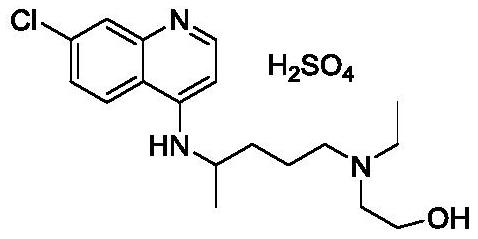
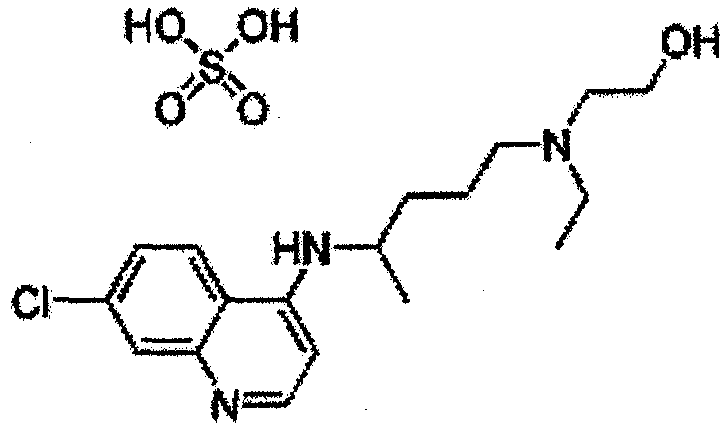
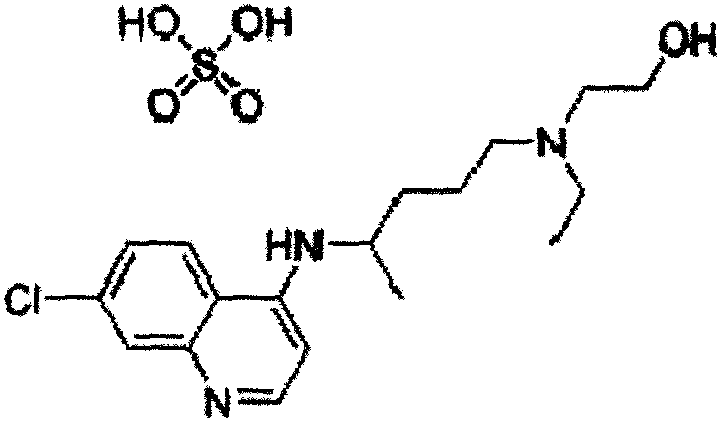
Hydroxychloroquine Sulfate is an antimalarial agent used for the treatment of systemic lupus erythematosus, rheumatoid arthritis and other autoimmune, inflammatory and dermatologic conditions. Also acts as an inhibitor of autophagy and toll-like receptor (TLR) 7/9.
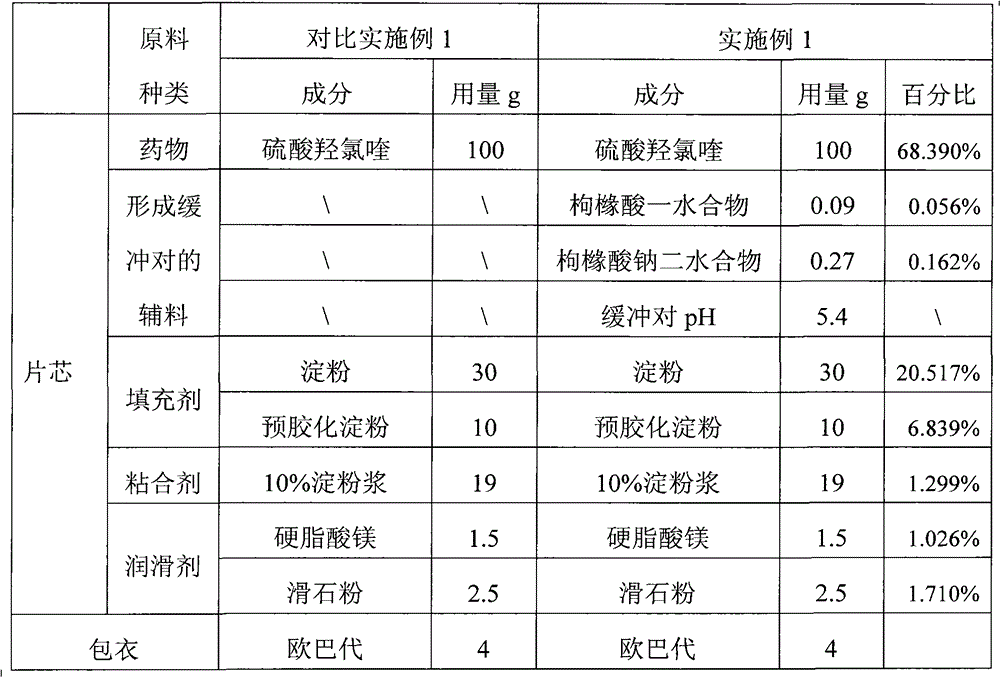
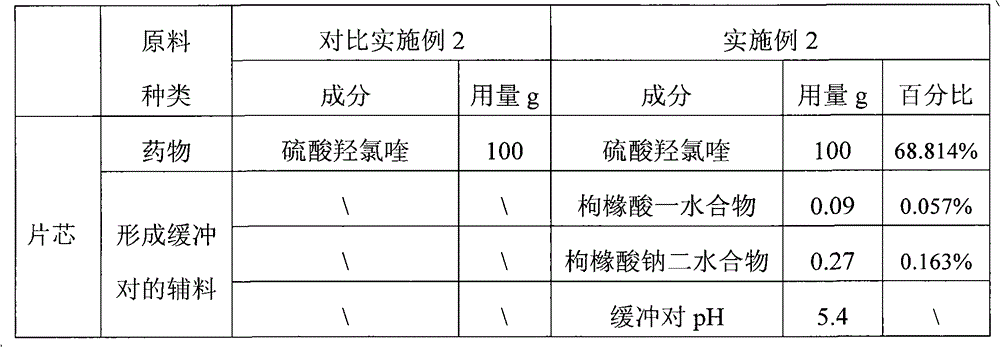
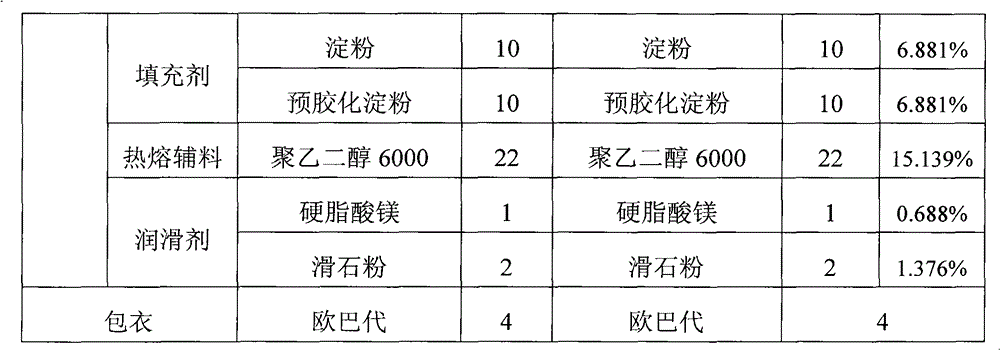
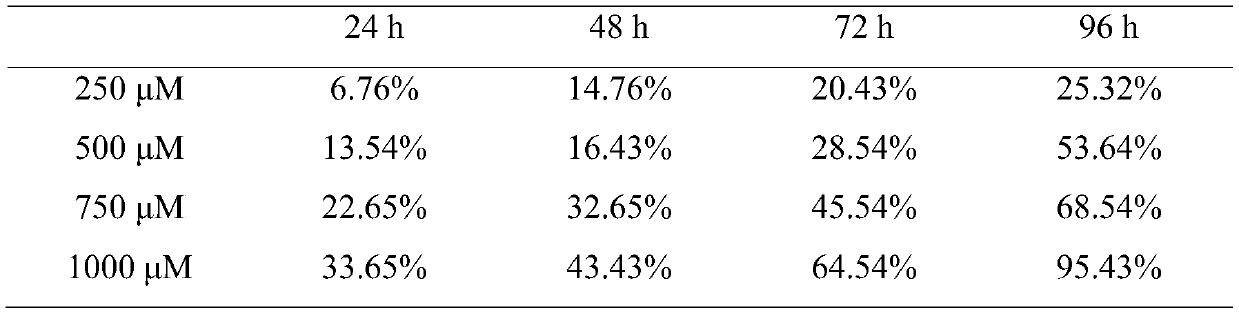
Hydroxychloroquine sulphate solid preparation and preparation method thereof
ActiveCN102525969BGood dissolution effectImprove stabilityOrganic active ingredientsAntipyreticBuffer solutionMedicinal chemistry
Owner:SHANGHAI ZHONGXI PHARMA
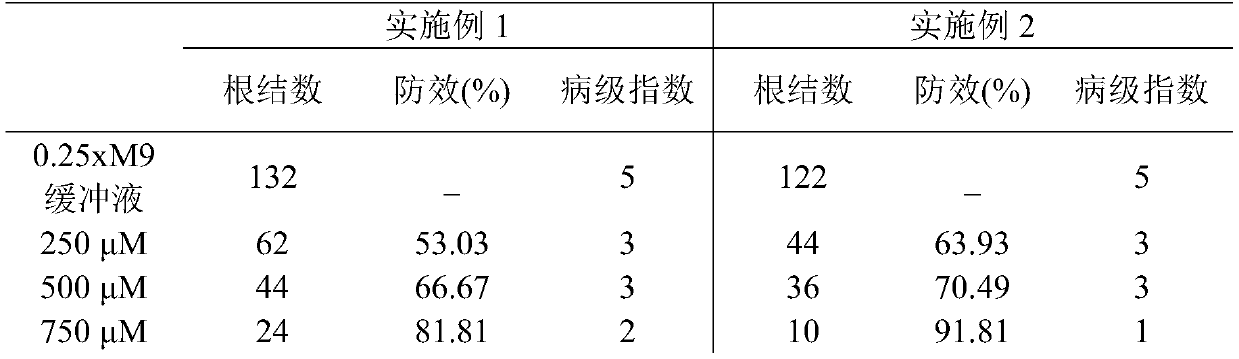
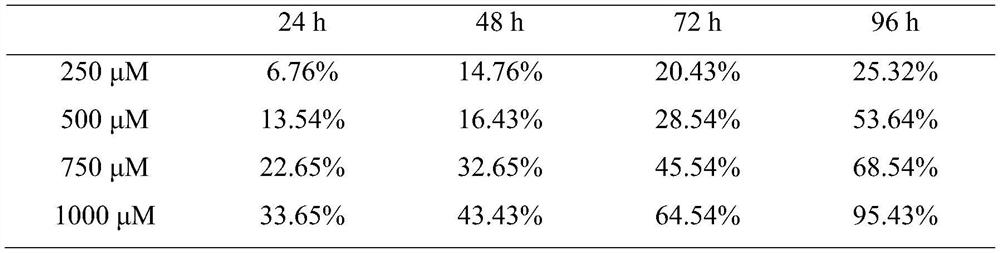
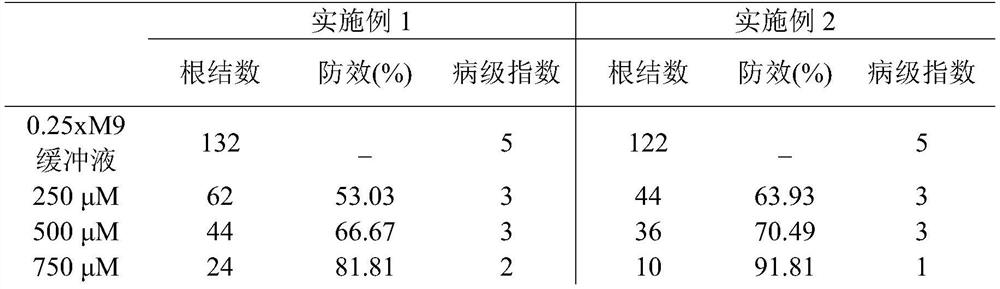
New use of chloroquine in prevention and control of root-knot nematodes
The invention discloses a new use of chloroquine in prevention and control of root-knot nematodes, and belongs to the technical field of the prevention and control of root-knot nematodes. The invention relates to a use of chloroquine in prevention and control of root-knot nematodes and a use of chloroquine in inhibition of infection, development and / or oviposition of root-knot nematodes, wherein the chloroquine is chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate. The invention also relates to a use of chloroquine in preparation of a root-knot nematode pesticide or a root-knot nematode infection, development and / or oviposition inhibitor, wherein the active component of the root-knot nematode pesticide or the root-knot nematode infection, development and / or oviposition inhibitor is chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate; and the root-knot nematode pesticide or the root-knot nematode infection, development and / or oviposition inhibitor is an aqueous solution of chloroquine phosphate, hydroxychloroquine sulfate or quinine sulfate. According to the present invention, the prevention and control of root-knot nematodes with the aqueous solution of chloroquine are safe, non-toxic and effective.
Owner:YUNNAN UNIV
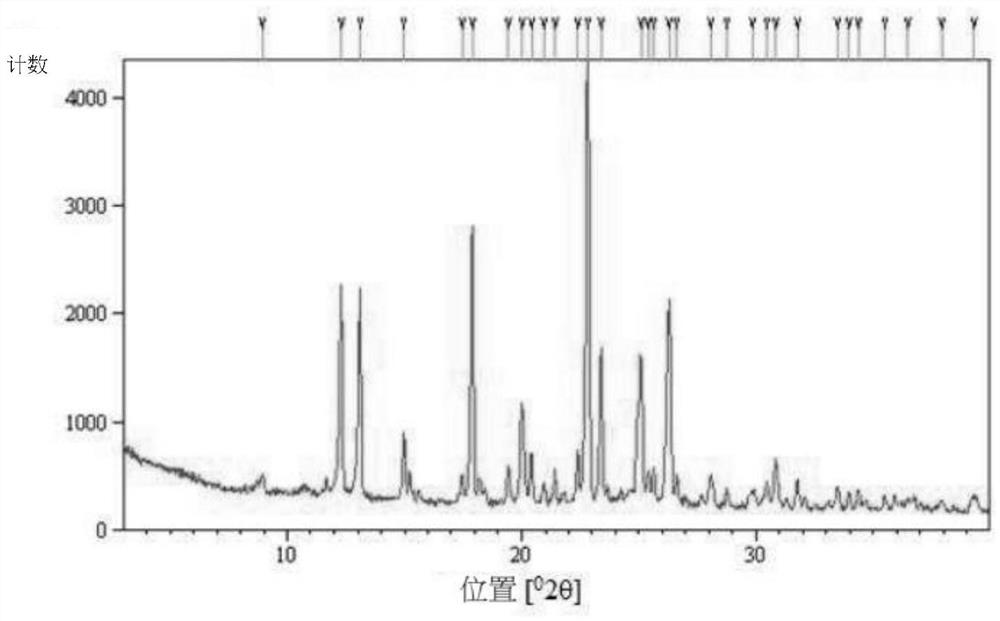
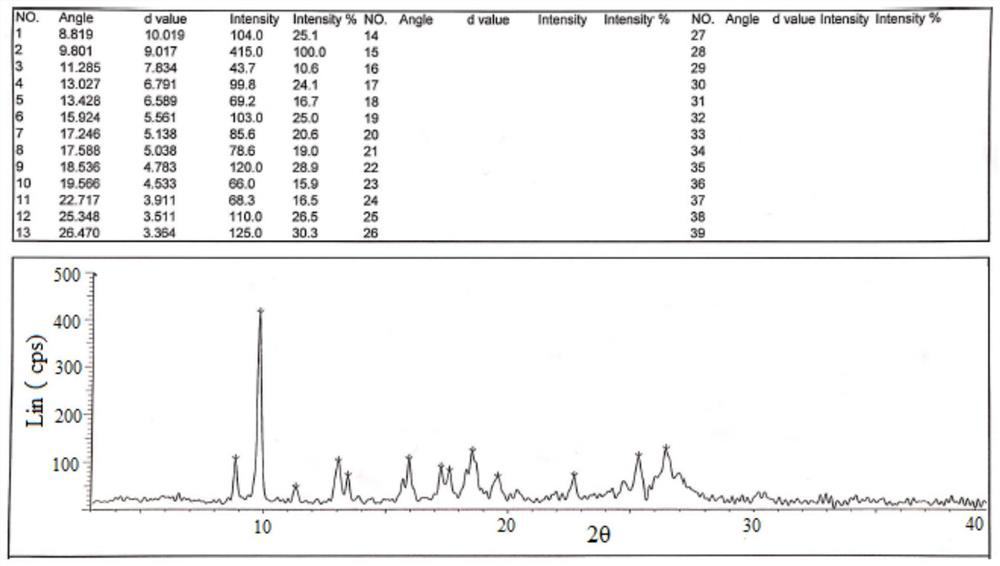
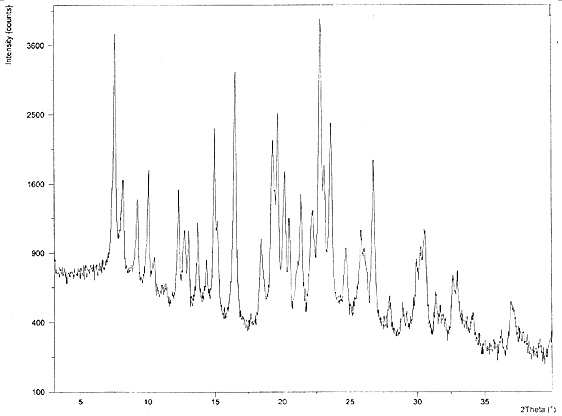
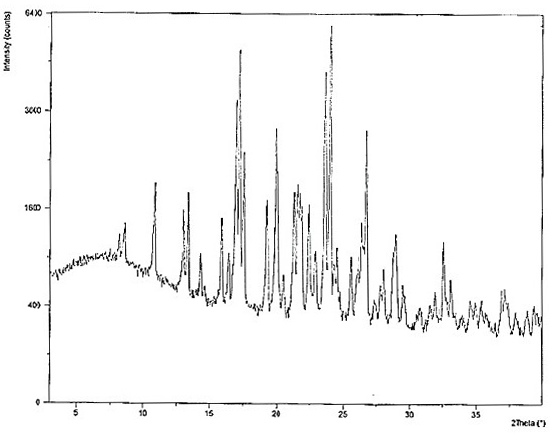
Crystals of hydroxychloroquine sulfate
PendingCN113527202AOrganic active ingredientsOrganic chemistry methodsHydroxychloroquine SulfateCondensed matter physics
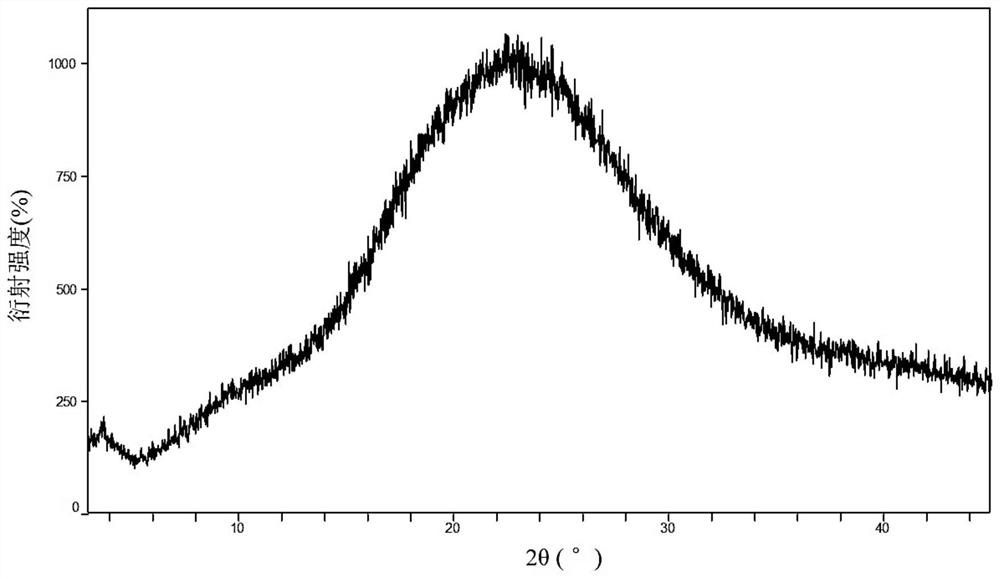
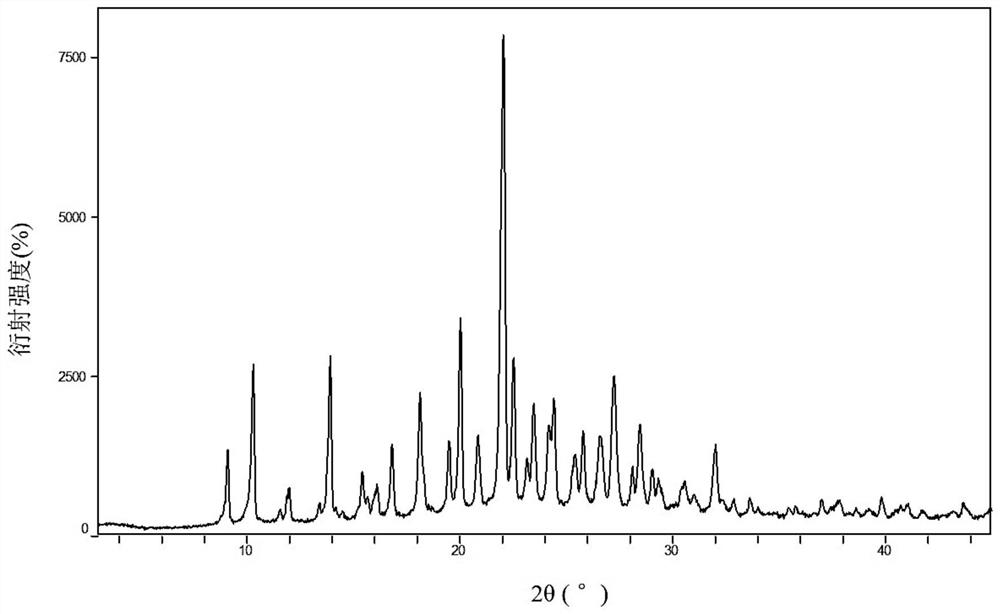
Two crystals of (S)-(+)-hydroxychloroquine sulfate. One crystal features diffraction peaks at 12.3 + / - 0.1 degrees, 13.1 + / - 0.1 degrees, 17.9 + / - 0.1 degrees, 22.8 + 0.1 degrees, 23.4 + 0.1 degrees, 25.1 + / - 0.1 degrees, and 26.3 + / - 0.1 degrees as 2theta angles in a powder X-ray diffraction pattern. The other crystal features diffraction peaks at 12.8 + / - 0.1 degrees, 14.5 + / - 0.1 degrees, 16.7 + 0.1 degrees, 17.6 + 0.1 degrees, 20.2 + / - 0.1 degrees, 21.4 + / - 0.1 degrees, 23.8 + / - 0.1 degrees, 25.7 + / - 0.1 degrees, and 26.0 + / - 0.1 degrees as 2theta angles in a powder X-ray diffraction pattern. Also disclosed are methods of preparing crystals of (S)-(+)-hydroxychloroquine sulfate.
Owner:GENELABS TECH INC
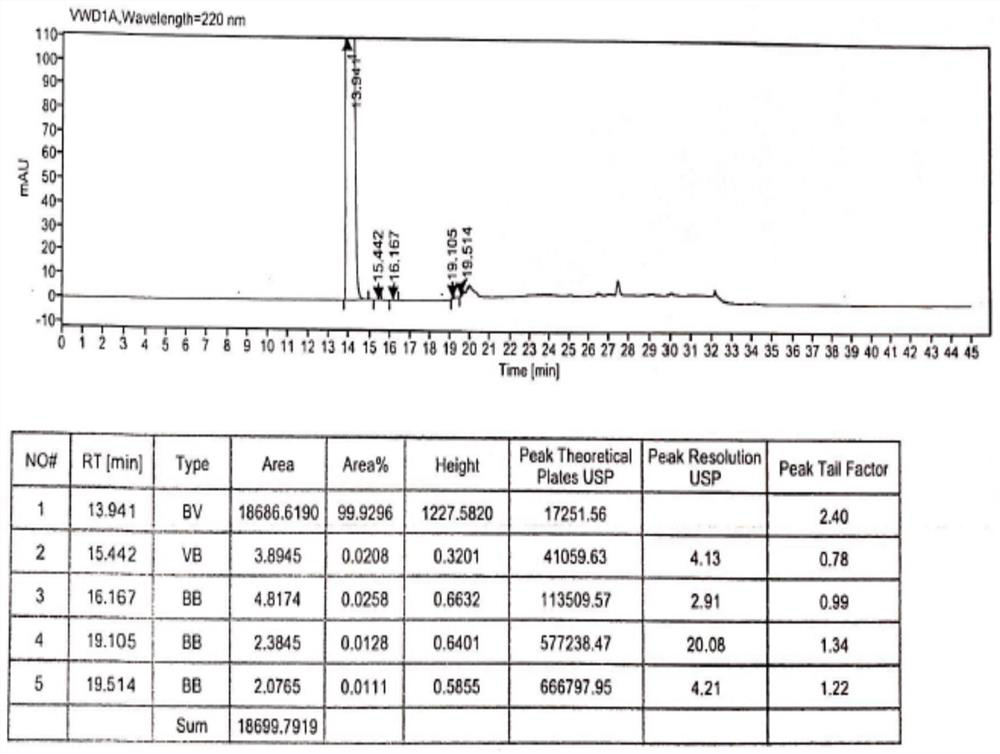
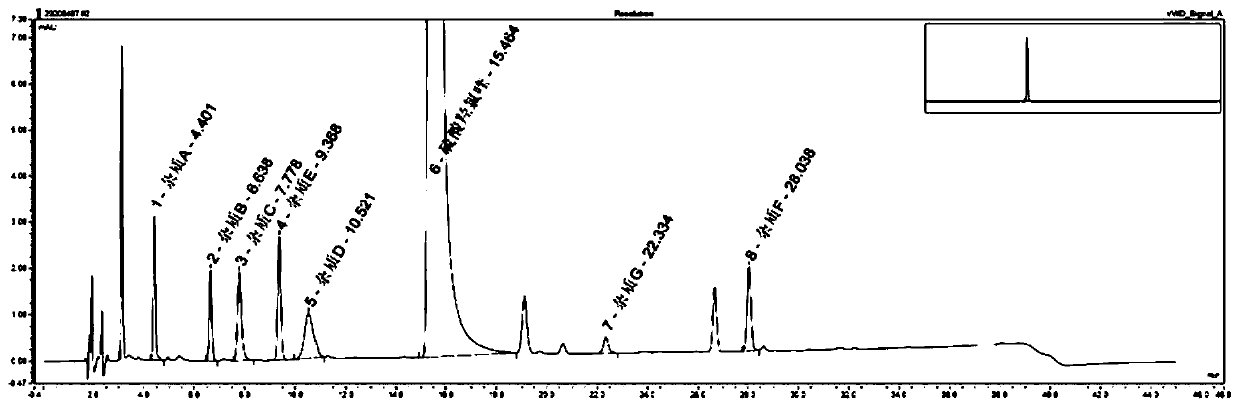
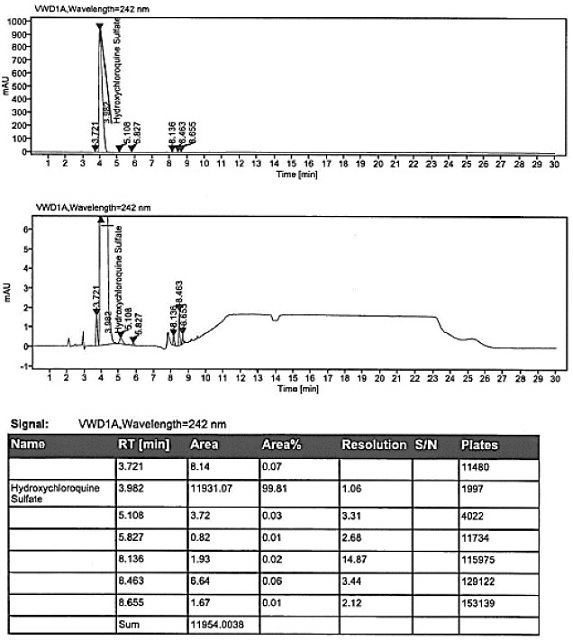
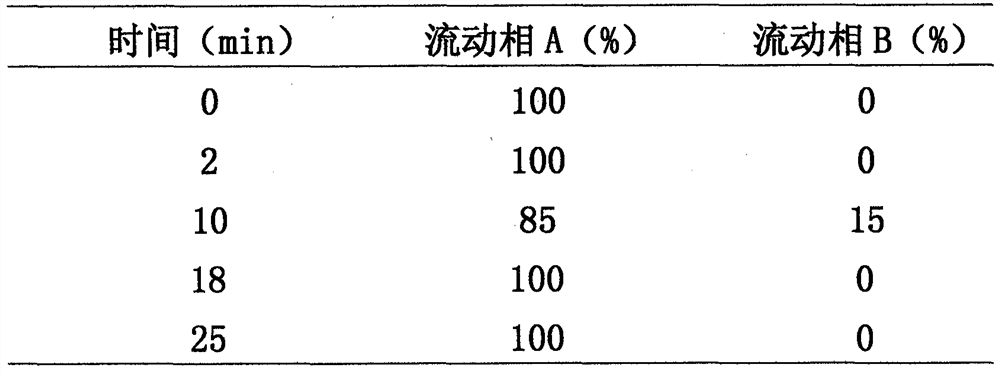
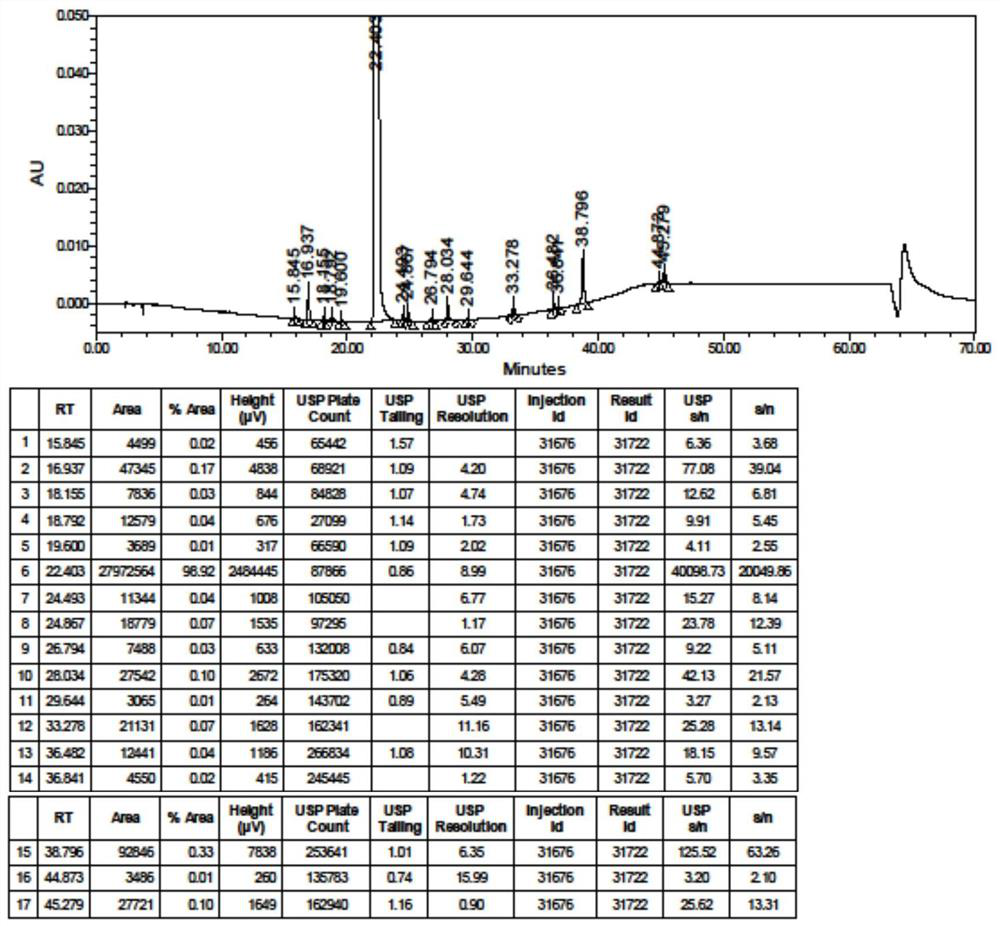
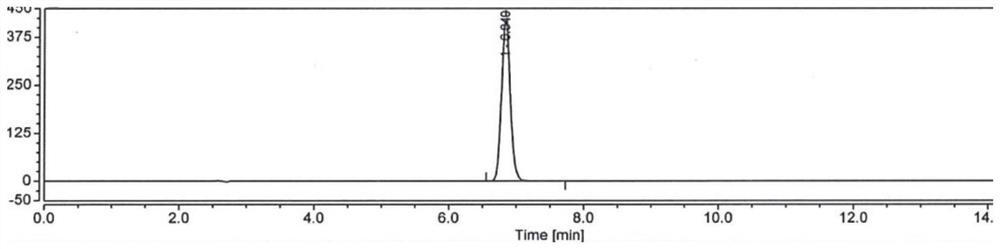
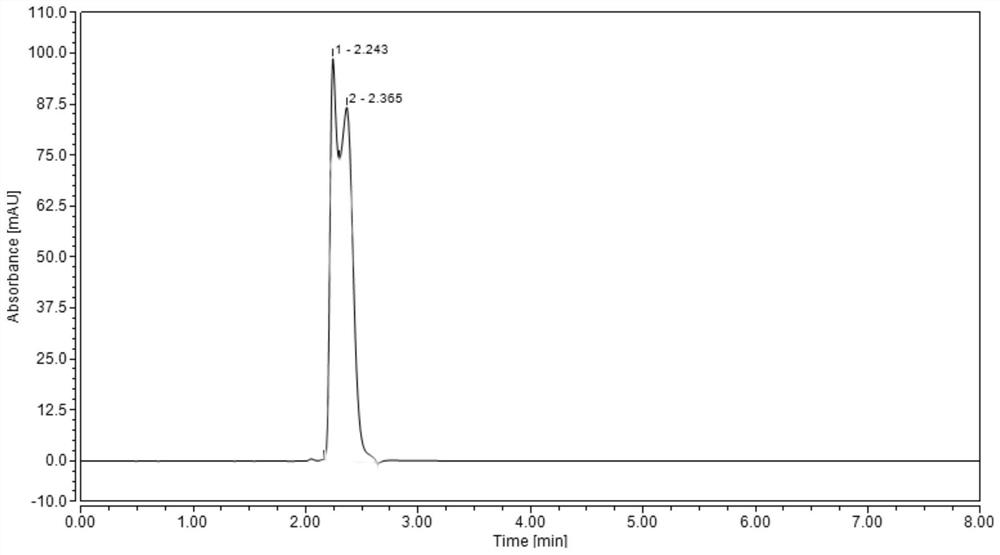
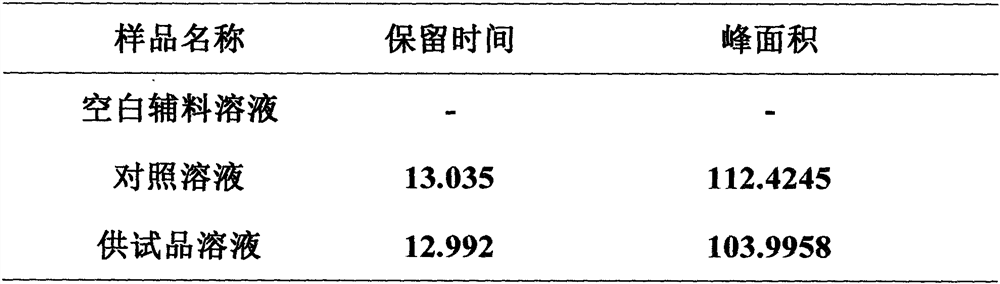
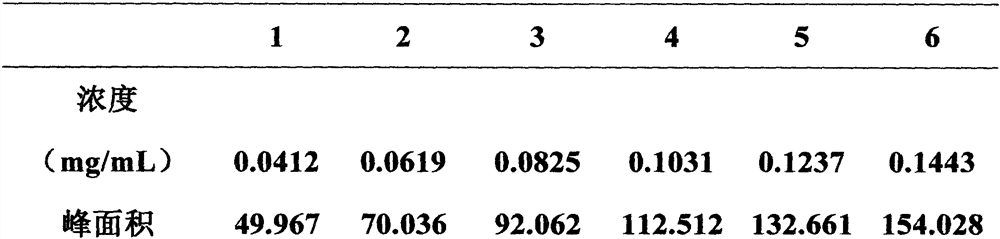
Method for analyzing composition of hydroxychloroquine sulfate preparation by high performance liquid chromatography
PendingCN111398475AImprove accuracyEasy to separateComponent separationHydroxychloroquineHydroxychloroquine Sulfate
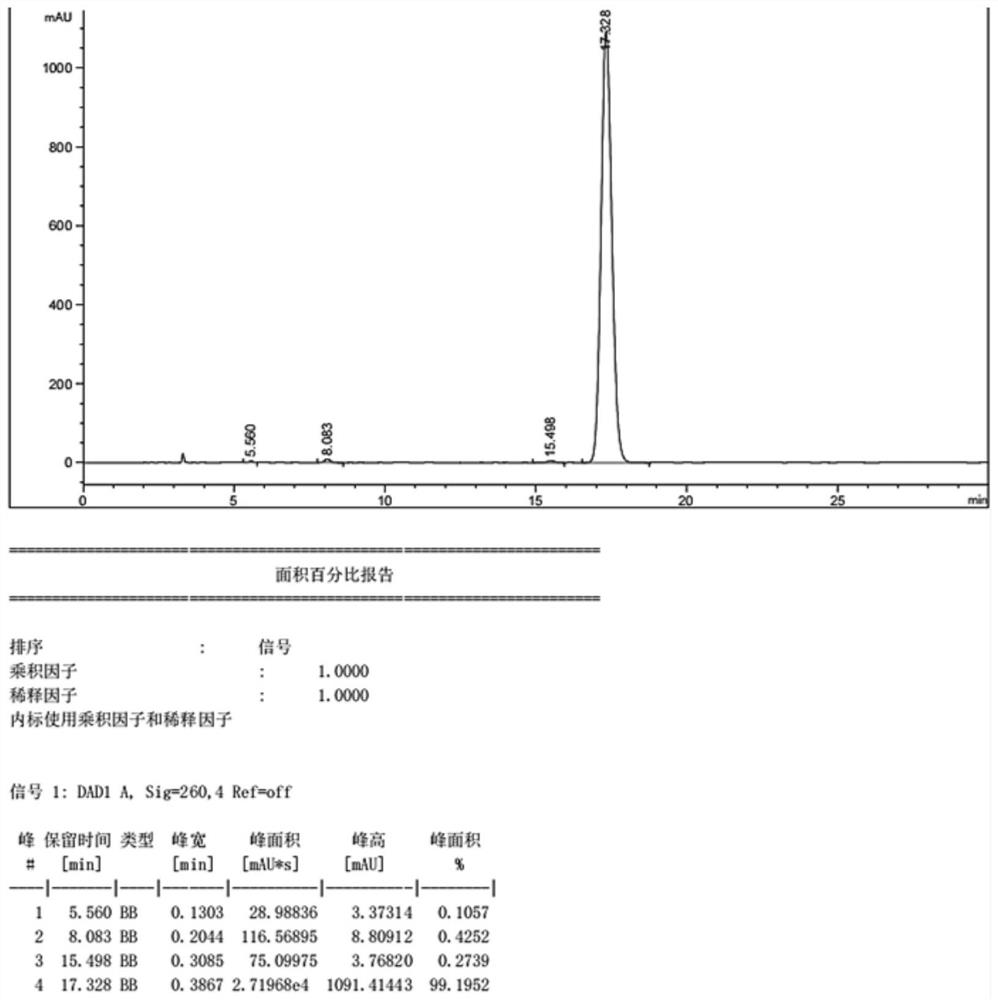
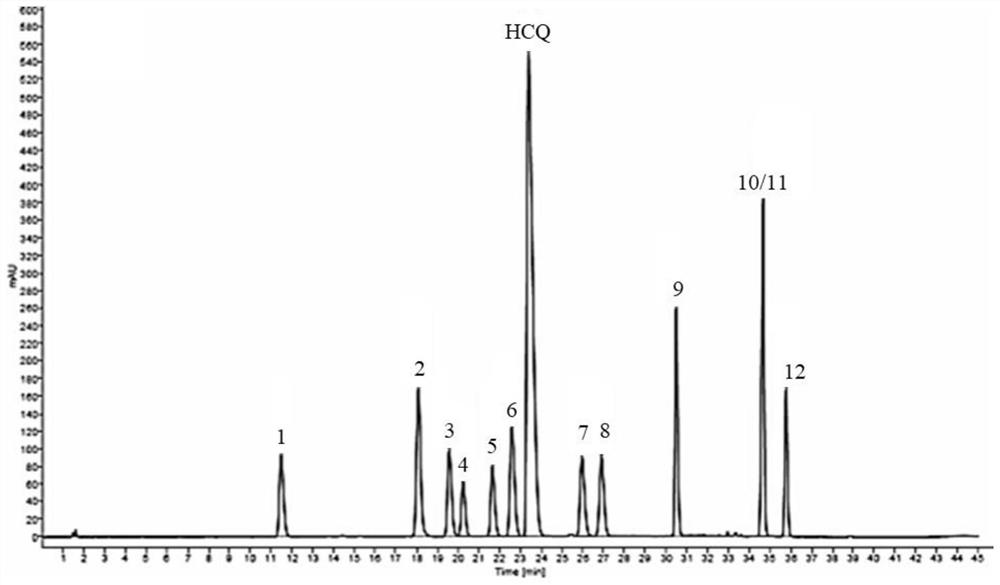
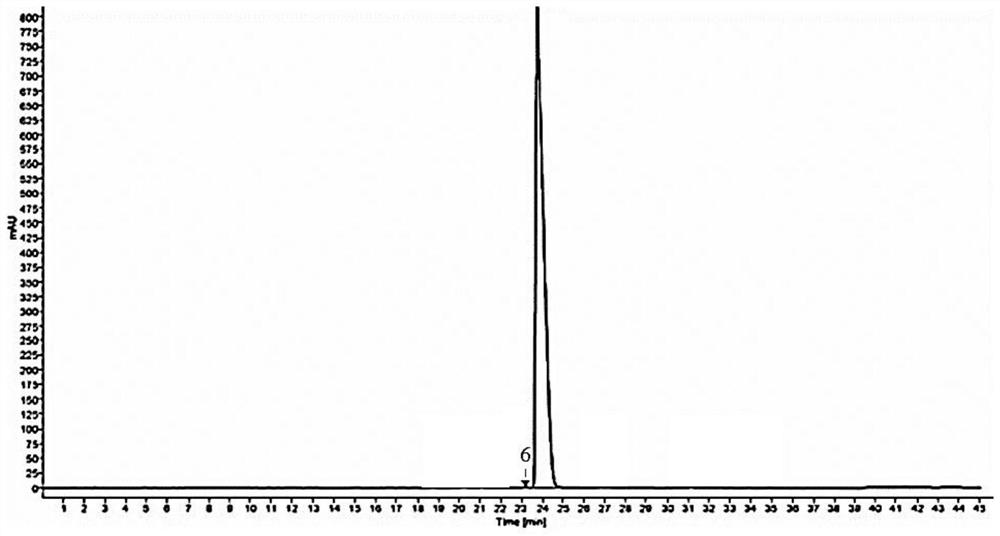
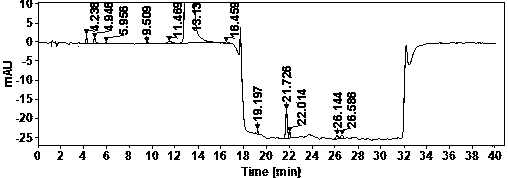
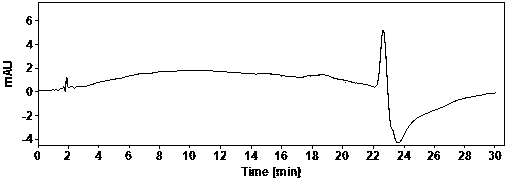
The invention discloses a method for analyzing a composition of a hydroxychloroquine sulfate preparation by high performance liquid chromatography. The method comprises the following steps: a. selecting 50mg of hydroxychloroquine sulfate reference substance, and precisely weighing; b, precisely weighing 1.0 ml, 2.0 ml, 3.0 ml, 4.0 ml, 5.0 ml, 6.0 ml and 7.0 ml of the mixed solutions obtained in the step a, and putting the mixed solutions into a 10ml volumetric flask for later use; c, performing chromatographic analysis by using a chromatographic column to obtain a regression equation of a concentration C and a peak area A; and d, removing a proper amount of a mixture obtained in the step a, precisely weighing, adding a diluent to dissolve, and quantitatively diluting to prepare a solutioncontaining 0.5 mg per 1ml to obtain a test solution; and precisely measuring the test solution, adding a mobile phase, diluting to prepare a solution containing 0.05 g per 1ml as a control solution, precisely measuring 0.2 ml of the control solution, injecting the control solution into a liquid chromatograph, and adjusting sensitivity of an instrument for testing. The method for analyzing the composition of the hydroxychloroquine sulfate preparation by the high performance liquid chromatography is simple and convenient to operate, sensitive, high in accuracy, suitable for clinical hydroxychloroquine serum drug concentration monitoring and high in specificity.
Owner:NANJING MEIRUI PHARMA CO LTD
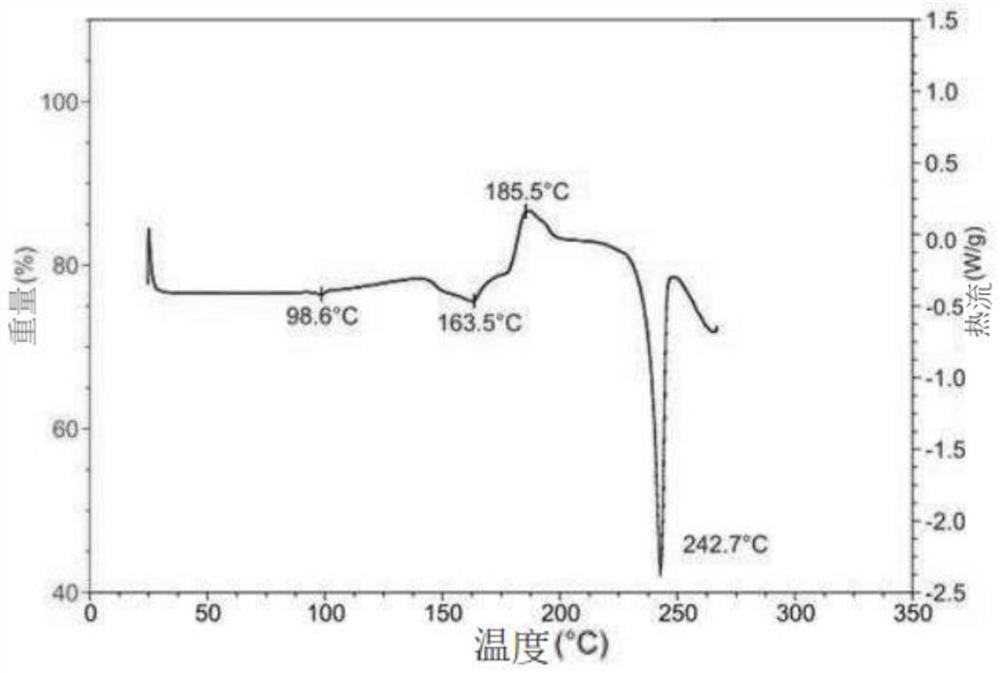
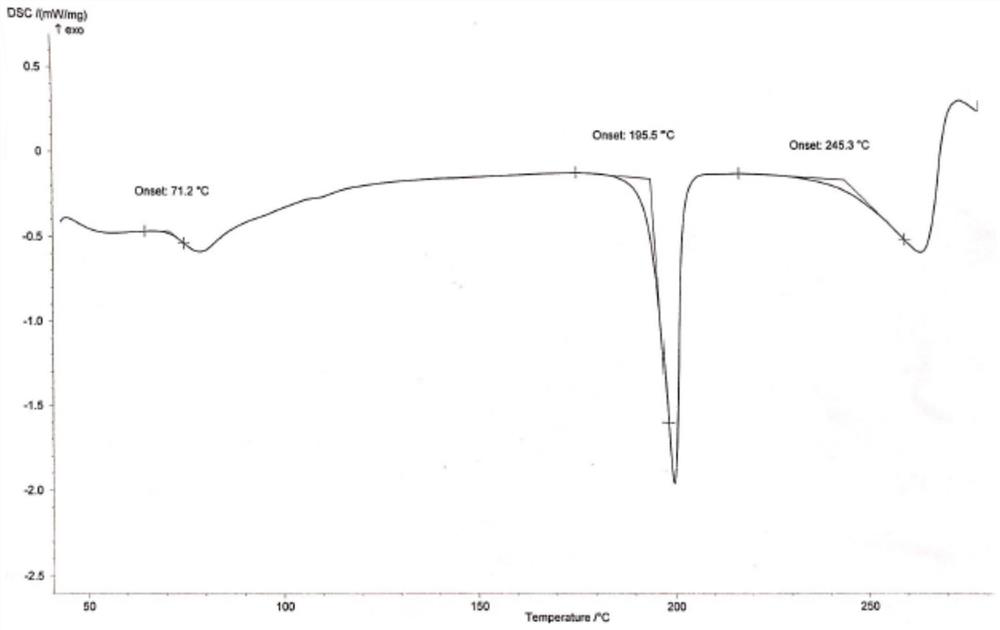
Hydroxychloroquine sulfate crystal form B and preparation method thereof
InactiveCN112480000AEasy to prepareLow hygroscopicityOrganic active ingredientsOrganic chemistry methodsPharmaceutical drugCombinatorial chemistry
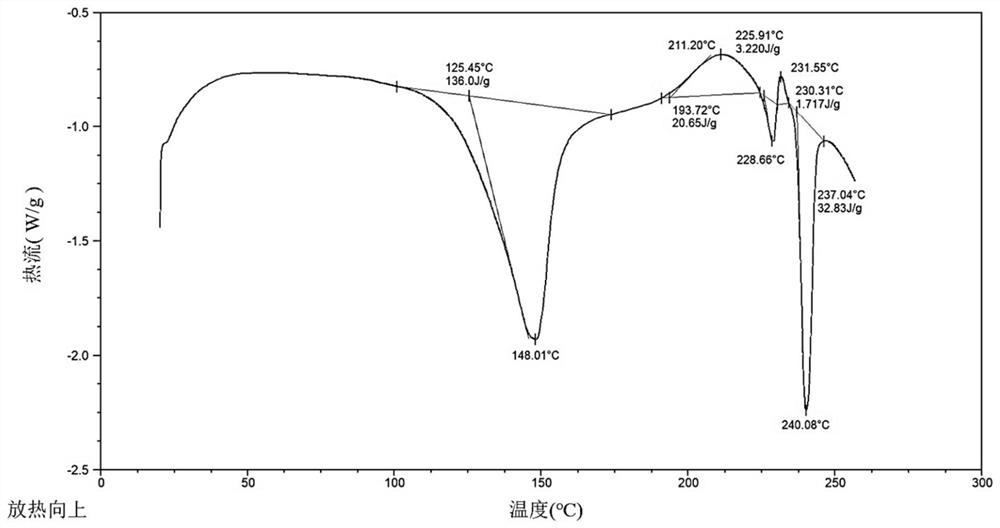
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a hydroxychloroquine sulfate crystal form B and a preparation method thereof, and XRD and DSC are usedfor characterization. The hydroxychloroquine sulfate crystal form B provided by the invention is simple in preparation method, low in hygroscopicity, good in stability, capable of forming a regular crystal form and higher in solubility, thereby being beneficial to process treatment of medicines, improvement of physical and chemical properties and improvement of patent medicine properties.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
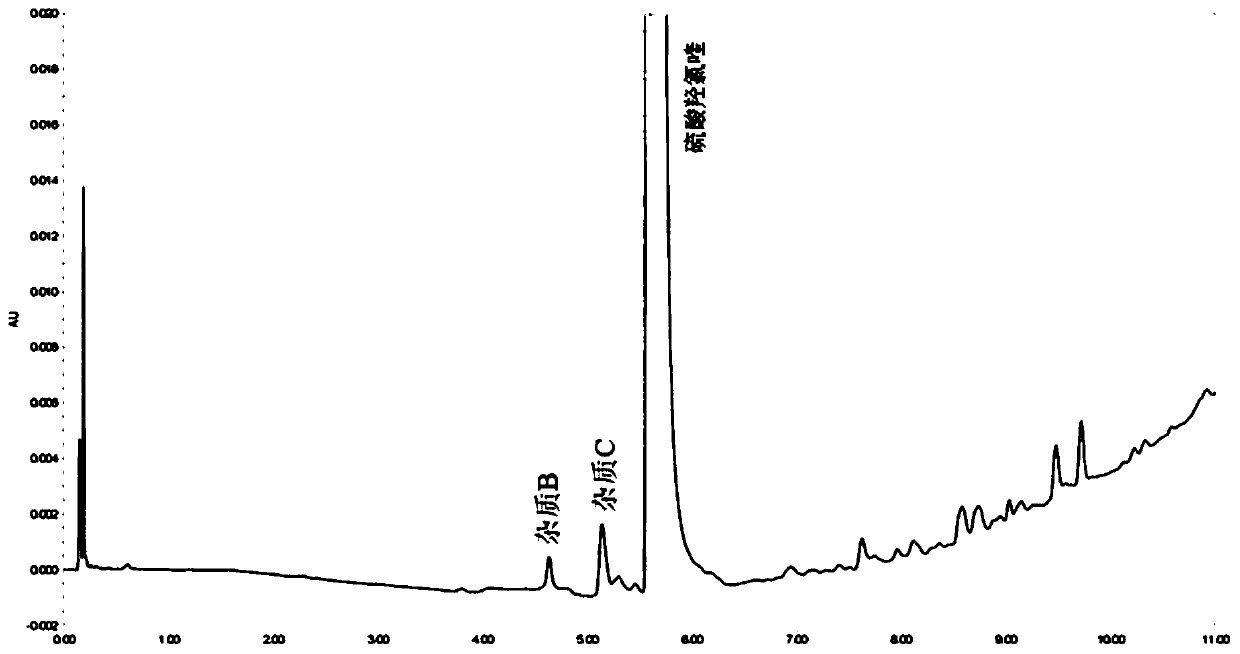
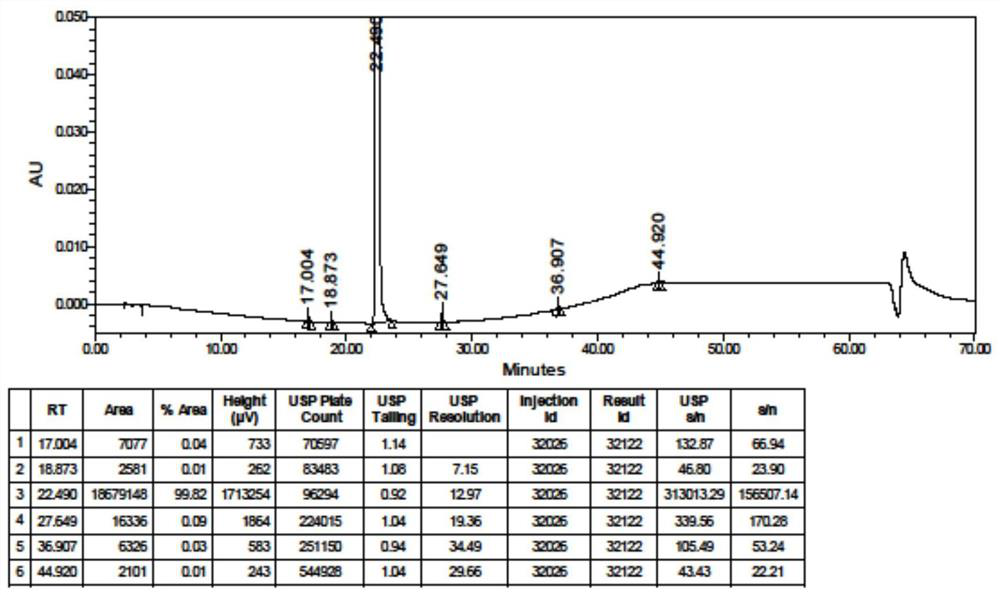
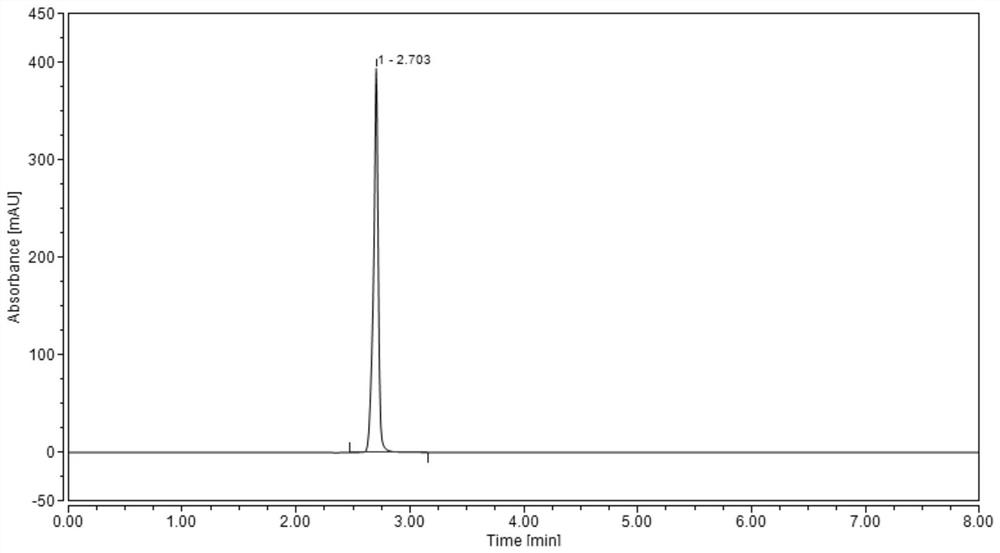
Method for detecting hydroxychloroquine sulfate related substances and application thereof
ActiveCN111551645AEasy to separateHigh sensitivityComponent separationHydroxychloroquine SulfateSolvent
The invention discloses a method for detecting hydroxychloroquine sulfate related substances. The method comprises the following step: carrying out separation and detection by adopting reverse liquidchromatography, wherein chromatographic conditions are as follows: a mobile phase is a mixed solvent consisting of a water-soluble organic solvent and a buffer solution; wherein the buffer solution isan ammonium acetate solution or an ammonium formate solution; wherein the pH value of the buffer solution is 9.0 to 10.5. The detection method disclosed by the invention can be used for simultaneously detecting seven impurities recorded in quality standards of hydroxychloroquine sulfate raw materials in European Pharmacopoeia 9.8 versions in hydroxychloroquine sulfate raw materials and preparations, and has the advantages of good separation effect, high sensitivity, no blank interference, low detection cost, high analysis speed and the like.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
Method for prevention and treatment of a viral-mediated infectious disease
A method for prevention or treatment of a viral-mediated infectious disease in a mammal. The mammal may be a human being. A therapeutic dose of a composition is administered, via an inhalation delivery apparatus, to the mammal. The composition includes microparticles and / or nanoparticles. The microparticles and / or nanoparticles include a first pharmaceutically active agent and a second pharmaceutically active agent. The first pharmaceutically active agent includes unfractionated heparin (UFH), Low Molecular Weight Heparin (LMWH), sulfated non-anticoagulant heparin (S-NACH) or combinations thereof. The second pharmaceutically active agent includes 10-30 mg of hydroxychloroquine in a form of hydroxychloroquine sulfate, 10-30 mg of favipiravir, or a combination thereof. The viral-mediated infectious disease is caused by one or more viruses in the mammal.
Owner:VIROTHERA PHARM LLC
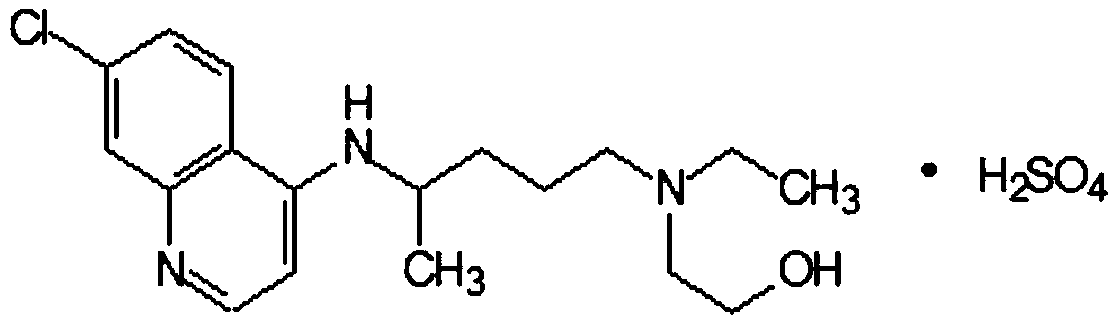
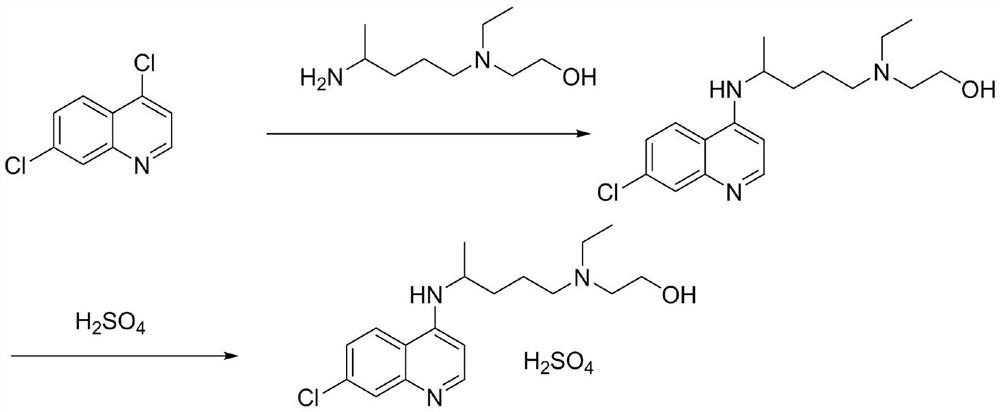
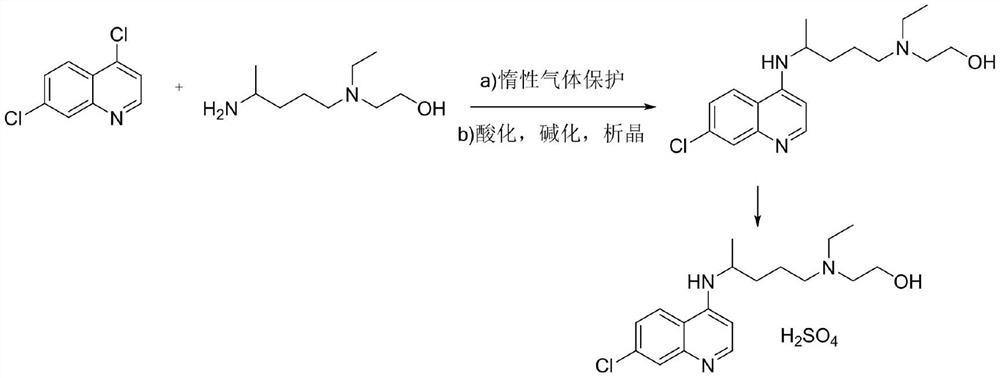
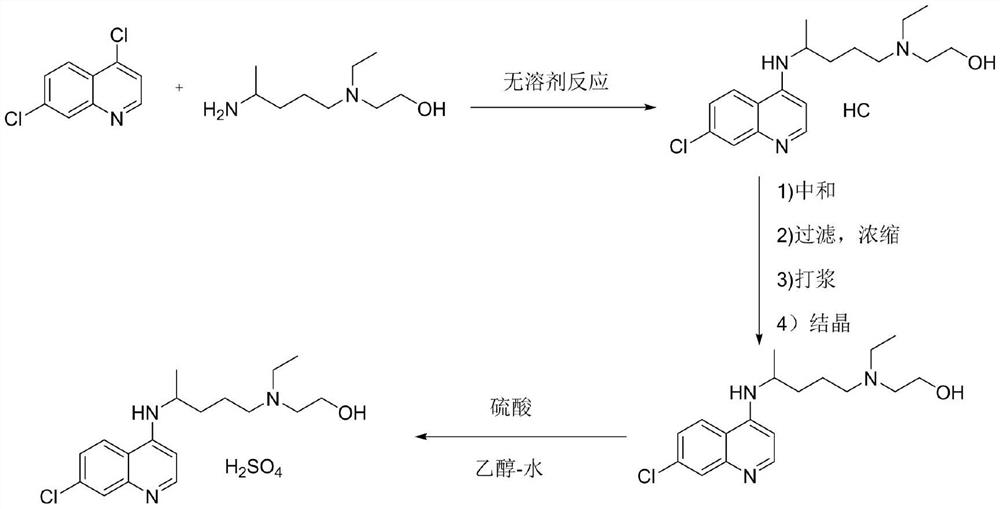
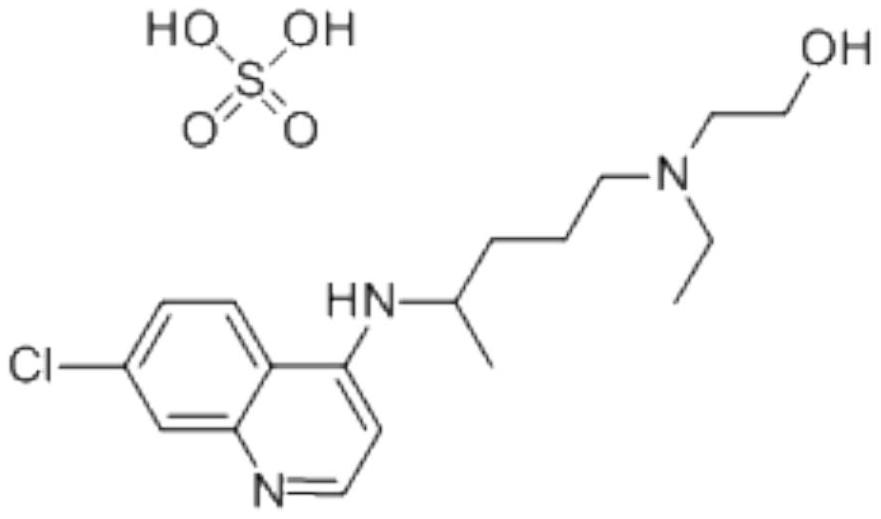
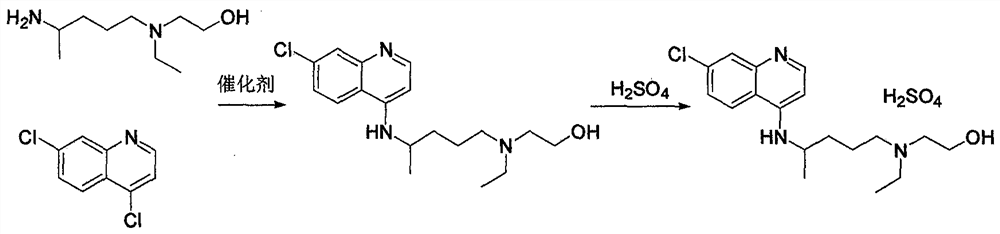
Hydroxychloroquine sulfate and preparation method thereof
PendingCN113185459AGood pollution effectSpeed up the reaction processOrganic chemistryHydroxychloroquinePtru catalyst
The invention discloses hydroxychloroquine sulfate and a preparation method thereof, and relates to the technical field of medicinal chemistry. The preparation method comprises the steps of mixing 4, 7-dichloroquinoline with a hydroxychloroquine side chain, carrying out heating condensation in the presence of an organic base catalyst, adding water and liquid, and carrying out cooling crystallization to obtain hydroxychloroquine; and dissolving hydroxychloroquine in an ethyl acetate and ethanol aqueous solution, heating, dissolving and clarifying, dropwise adding concentrated sulfuric acid, cooling, crystallizing, filtering and drying to obtain hydroxychloroquine sulfate. The method has the beneficial effects that a solvent-free reaction is used, and a catalyst is added, so that high pollution is avoided, the reaction process is accelerated, and the operation is simple; and meanwhile, the HPLC purity of the obtained hydroxychloroquine refined product is not less than 96.50%, the maximum single impurity content is less than 0.10%, the yield can reach 85%, the HPLC purity of hydroxychloroquine sulfate is not less than 98.00%, the maximum single impurity content is less than 0.10%, and the yield can reach 90%.
Owner:JIANGXI GUOYAO PHARMA LLC +1
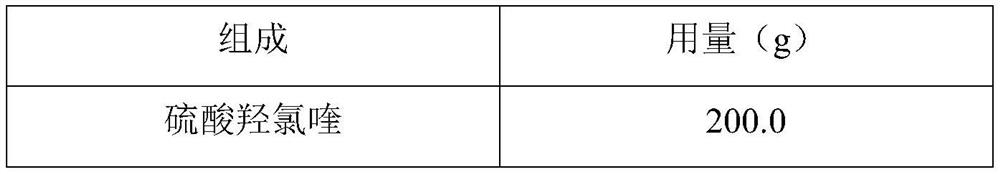
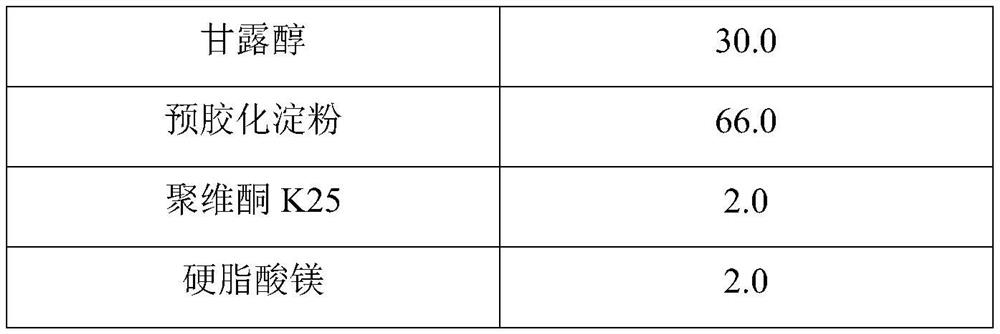
Hydroxychloroquine sulfate pharmaceutical preparation
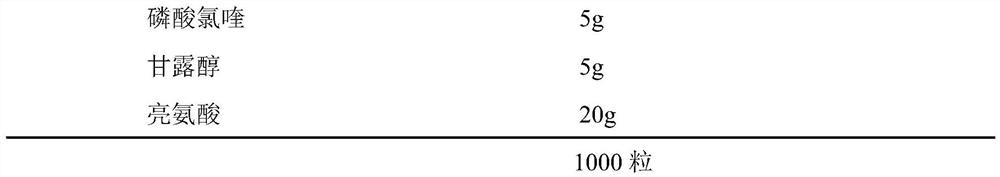
PendingCN113244180ASolve the problem of easy stickingSolve the problem of poor compressibility caused by high friabilityOrganic active ingredientsAntipyreticDrugs preparationsHydroxychloroquine Sulfate
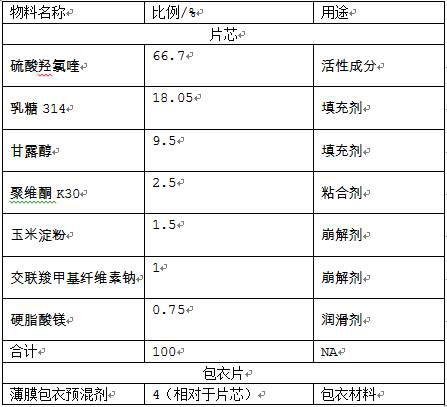
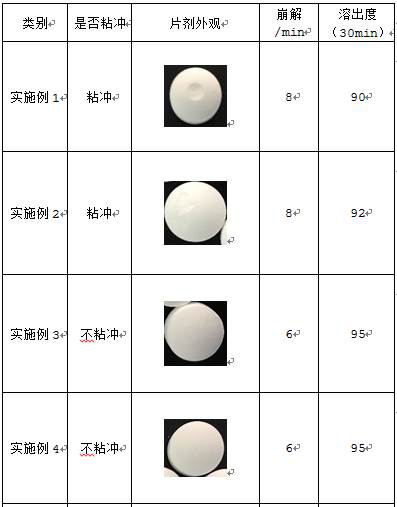
The invention provides a hydroxychloroquine sulfate pharmaceutical preparation. The hydroxychloroquine sulfate pharmaceutical preparation comprises the following components in percentage by weight: 50-75% of hydroxychloroquine sulfate, 5-15% of mannitol, 15-35% of pregelatinized starch, 0.5-2.0% of a water-soluble adhesive and 0.5-2.0% of a lubricant. According to the hydroxychloroquine pharmaceutical preparation disclosed by the invention, the mannitol and the pregelatinized starch are used as filling agents, and are matched with the adhesive and the lubricant, so that the problem that the hydroxychloroquine pharmaceutical preparation is extremely likely to stick and the problem of poor compressibility caused by high friability of tablets can be solved, the hydroxychloroquine pharmaceutical preparation has good dissolution property, and the dissolution effect is consistent with that of a reference reagent.
Owner:BEIJING WINSUNNY PHARMA CO LTD
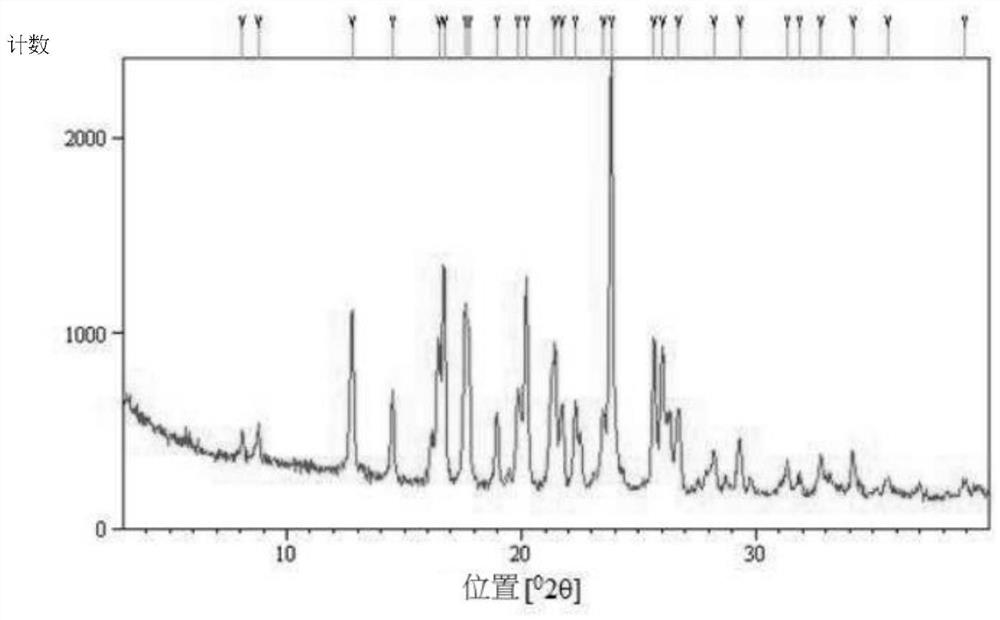
Hydroxychloroquine sulfate as well as crystal form and preparation method of enantiomer of hydroxychloroquine sulfate
PendingCN111793026AGood chemical stabilityReduce riskOrganic compound preparationOrganic chemistry methodsNitrogen oxidesEnantiomer
The invention relates to hydroxychloroquine sulfate as well as a crystal form and a preparation method of an enantiomer of hydroxychloroquine sulfate. The invention provides a crystal form A hydroxychloroquine sulfate. An X-ray powder diffraction pattern of the crystal form A hydroxychloroquine sulfate has characteristic peaks at 10.8 degrees, 13.0 degrees, 13.3 degrees, 16.9 degrees, 17.2 degrees, 17.5 degrees, 19.9 degrees, 21.3 degrees, 23.5 degrees, 24.0 degrees and 26.7 degrees + / -0.2 degrees, and does not contain hydroxychloroquine nitrogen oxide; the invention provides a hydroxychloroquine crystal, the X-ray powder diffraction pattern has characteristic peaks at 7.5 degrees, 14.9 degrees, 16.5 degrees, 19.2 degrees, 19.6 degrees, 22.8 degrees, 23.6 degrees and 26.7 degrees+ / -0.2 degree; the invention provides an S-hydroxychloroquine sulfate monohydrate, the X-ray powder diffraction pattern has characteristic peaks at 12.2 degrees, 13.0 degrees, 14.9 degrees, 17.8 degrees, 22.7 degrees, 23.3 degrees, 25.0 degrees and 26.2 degrees + / -0.2 degrees; the invention also provides R-hydroxychloroquine sulfate, the X-ray powder diffraction pattern has characteristic peaks at 12.2 degrees, 13.0 degrees, 14.9 degrees, 17.8 degrees, 22.7 degrees, 23.3 degrees, 25.0 degrees and 26.2 degrees + / -0.2 degrees.
Owner:珠海润都制药股份有限公司
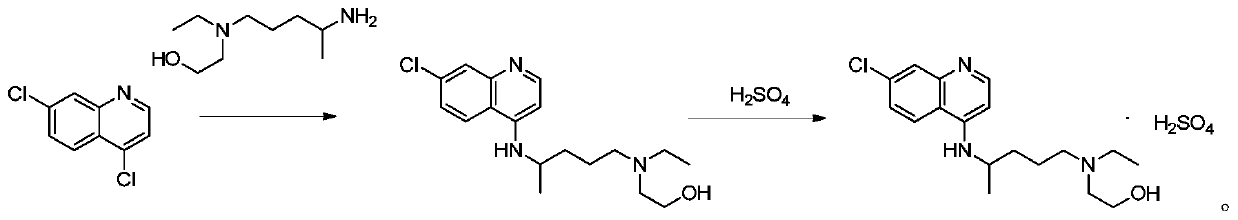
Preparation method of hydroxychloroquine sulfate
PendingCN111423373ASimple processGood reproducibilityOrganic chemistryHydroxychloroquineReaction temperature
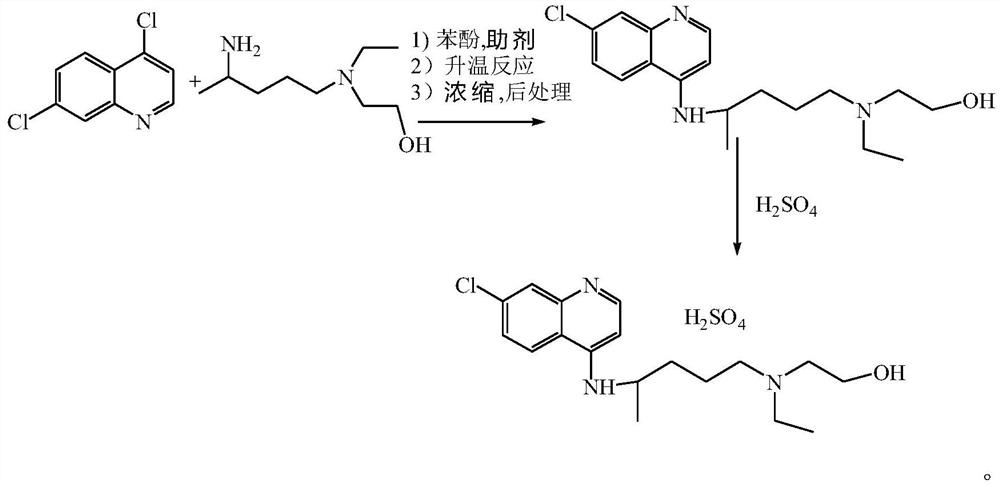
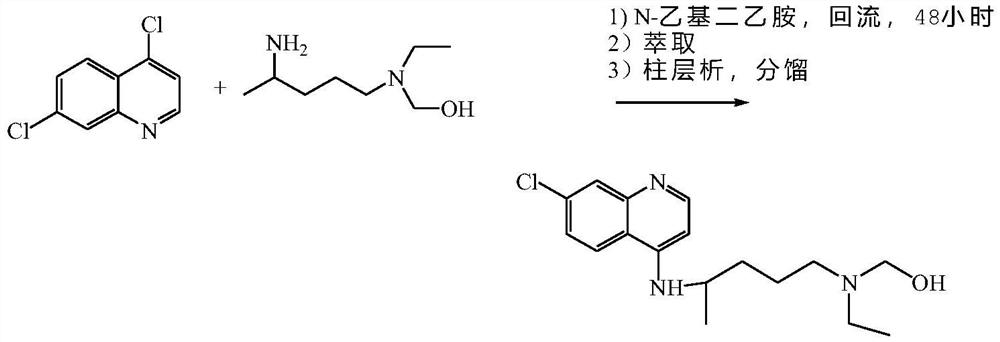
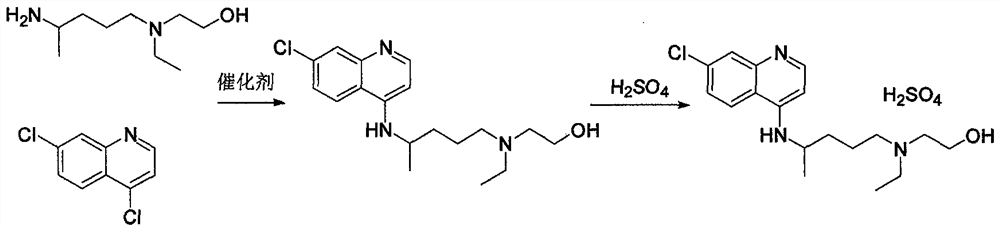
The invention relates to a preparation method of hydroxychloroquine sulfate, and belongs to the technical field of medicine synthesis. According to the preparation method of hydroxychloroquine sulfate, 7-chloro-4-fluoroquinoline and 5-(N-ethyl-N-2-hydroxyethyl amine)-2-pentylamine are subjected to a reaction to prepare hydroxychloroquine, and then hydroxychloroquine and sulfuric acid are salifiedto prepare hydroxychloroquine sulfate. The 7-chloro-4-fluoroquinoline is prepared from 4,7-dichloroquinoline through a halogen exchange reaction. The preparation method of hydroxychloroquine sulfate has the advantages of low reaction temperature, short reaction time, fewer byproducts, simple technique and favorable reproducibility, and is beneficial to industrial production.
Owner:REYOUNG PHARMA
Purification method of hydroxychloroquine sulfate
InactiveCN111377861AImprove the purification effectSimple and safe operationOrganic chemistryHydroxychloroquine SulfateSolvent
The invention discloses a purification method of hydroxychloroquine sulfate, which comprises the following steps: (1) dissolving a hydroxychloroquine sulfate crude product in water or a mixed solventof water and a first solvent to obtain a mixed solution I; (2) adding a second solvent into the mixed solution I, and carrying out reflux crystallization; and (3) cooling the reaction product, and filtering and drying the reaction product to obtain a fine hydroxychloroquine sulfate product. The method is excellent in purification effect, so that hydroxychloroquine sulfate with the purity of 99.5%or above and the particle size Dx (90) larger than or equal to 200 microns is prepared. The method is easy and convenient to operate, low in cost, safe, environmentally friendly and suitable for large-scale production.
Owner:CINKATE PHARMA INTERMEDIATES +1
Hydroxychloroquine sulfate sustained release microsphere for articular cavity injection and preparation method thereof
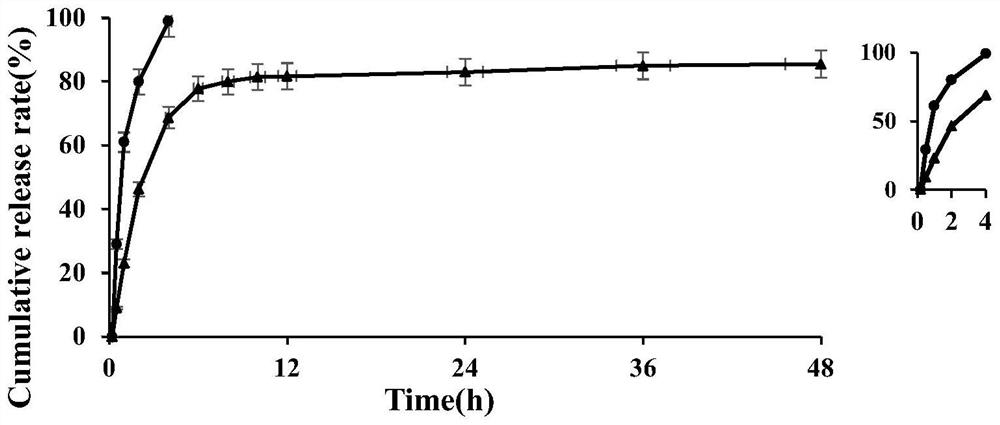
ActiveCN114796126AFlat shapeUniform particle size distributionOrganic active ingredientsAntipyreticHydroxychloroquineMicrosphere
The invention discloses a hydroxychloroquine sulfate sustained-release microsphere for articular cavity injection and a preparation method thereof. The preparation method comprises the following steps: dissolving a surfactant in an organic solvent to form an oil phase; adding hydroxychloroquine sulfate and gelatin into water to obtain a water phase; dropwise adding the water phase into the oil phase, stirring and emulsifying to form a W / O type emulsion; transferring the W / O emulsion into an ice bath, and adding a curing agent for cross-linking curing; and then adding isopropanol for dehydration, suction filtration, washing and drying to obtain the hydroxychloroquine sulfate sustained-release microspheres for articular cavity injection. The prepared hydroxychloroquine microspheres can stably and continuously release drugs for more than 48 hours, a sustained-release preparation is obtained, the administration frequency can be reduced, the total dosage can be reduced, the compliance of patients can be improved, and the clinical application potential is good.
Owner:复旦大学附属中山医院青浦分院(上海市青浦区中心医院)
Inhalant containing chloroquine therapeutic agent and preparation method of inhalant
InactiveCN113559086AImprove stabilityImprove the protective effectOrganic active ingredientsPowder deliveryOral medicationInhalation
The invention provides a chloroquine inhalant and a preparation method thereof, and belongs to the field of medicine preparations. The inhalant comprises chloroquine phosphate or hydroxychloroquine sulfate, a stabilizer and a diluent, can improve use efficiency, has a preventive effect, such as a new epidemic situation-COVID-19, and can take effect quickly. The powder inhalation has better stability, has the effects of masking taste, enhancing stability and protecting organism biological membranes on chloroquine phosphate or hydroxychloroquine sulfate after being wrapped by the stabilizer, is absorbed through the lungs, has a high targeting effect, also can effectively avoid the first-pass effect caused by the liver during oral administration, and improves bioavailability.
Owner:DISCOVERY SHENZHEN NEW MEDICINES DEV CO LTD
Preparation method of hydroxychloroquine sulfate
PendingCN114478377ASimple processing methodEasy to operateOrganic chemistryHydroxychloroquineSide chain
The invention discloses a preparation method of hydroxychloroquine sulfate, and belongs to the technical field of medicine synthesis. The method comprises the following steps: (1) mixing 4, 7-dichloroquinoline, an auxiliary agent and phenol, heating, adding a side chain, and reacting to obtain a hydroxychloroquine crude product; (2) purifying the hydroxychloroquine crude product obtained in the step (1) to obtain hydroxychloroquine; and (3) dissolving the hydroxychloroquine obtained in the step (2), and adding sulfuric acid to obtain hydroxychloroquine sulfate. The preparation method can shorten the reaction time, reduce the reaction temperature, reduce the generation of impurities, greatly reduce the production cost and reduce the environmental pollution, and is simple to operate and suitable for industrial production.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Preparation method of hydroxychloroquine
PendingCN112745263AHigh yieldHigh purityOrganic chemistryBulk chemical productionHydroxychloroquineQuinoline
The invention relates to a preparation method of hydroxychloroquine, which comprises the following steps: protecting hydroxyl of 5-(N-ethyl-N-ethoxyl)-2-aminopentane through a silanization reagent, removing amino protons from tetrahydrofuran or toluene by using a bis(trimethylsilyl lithium amide) solution to form amino anions, and carrying out a substitution reaction with 4.7 dichloroquinoline to generate hydroxychloroquine. The hydroxychloroquine and sulfuric acid are salified in an alcoholic solution to generate hydroxychloroquine sulfate, and the hydroxychloroquine sulfate preparation method provided by the invention has the characteristics of low toxicity, low pollution, high purity, low reaction temperature, short reaction time, high yield and the like, and is suitable for industrialization.
Owner:NANJING GRITPHARMA CO LTD
Preparation method of hydroxychloroquine sulfate impurity
PendingCN114380744AReasonable process designSynthetic operation is simpleOrganic chemistryDrugs synthesisPharmaceutical drug
The invention discloses a preparation method of a hydroxychloroquine sulfate impurity, and belongs to the field of drug synthesis, the preparation method provided by the invention is reasonable in process design, strong in operability, high in yield and capable of realizing mass production. 5-chloro-2-pentanone is taken as a raw material, synthesis of the hydroxychloroquine sulfate impurity is realized through electrophilic reaction, reductive amination, hydrogenation deprotection and nucleophilic substitution reaction, the target impurity prepared by the method provides an important basis for scientific evaluation of quality and safety of hydroxychloroquine sulfate, and the method is suitable for industrial production. The method is of great significance to reasonable medication of hydroxychloroquine sulfate.
Owner:SHENZHEN SUNGENING BIO-MEDICAL CO LTD
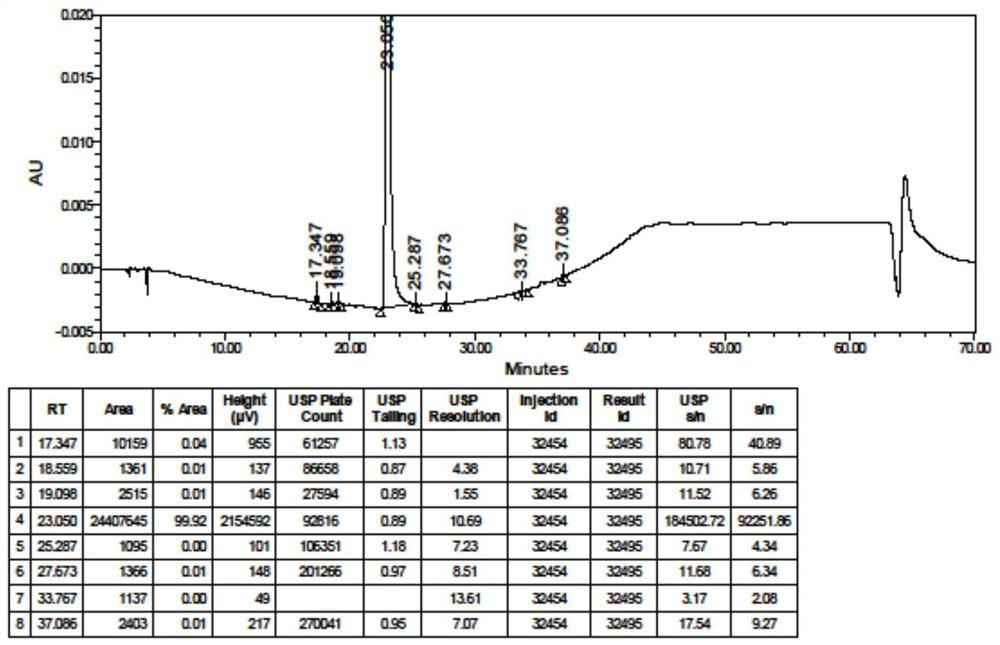
HPLC analysis method for hydroxychloroquine sulfate and related substances thereof
PendingCN114062520AHigh precisionImprove accuracyComponent separationPhysical chemistryHydroxychloroquine Sulfate
The invention discloses an HPLC analysis method for hydroxychloroquine sulfate and related substances thereof, which comprises the steps of dissolving a tested substance in a solution containing perchloric acid, and carrying out HPLC detection, wherein the chromatographic conditions are as follows: a mobile phase comprises a mobile phase A and a mobile phase B; the mobile phase A is a perchloric acid solution; the mobile phase B is acetonitrile; and the chromatographic column is a phenyl column. According to the method, hydroxychloroquine sulfate, 12 related substances and contents thereof can be detected at the same time, and effective separation of hydroxychloroquine sulfate and related substances thereof is realized; and the mobile phase system is simple and does not cause damage to an instrument or a chromatographic column, and the method is simple to operate, high in precision, good in reproducibility, high in sensitivity, high in accuracy and low in cost.
Owner:SHANGHAI ZHONGXI PHARMA +1
Method for detecting mixing uniformity of hydroxychloroquine sulfate particles
PendingCN113804642AAccurate detectionEfficient and convenient monitoringPreparing sample for investigationMaterial analysis by optical meansPhysical chemistryHydroxychloroquine Sulfate
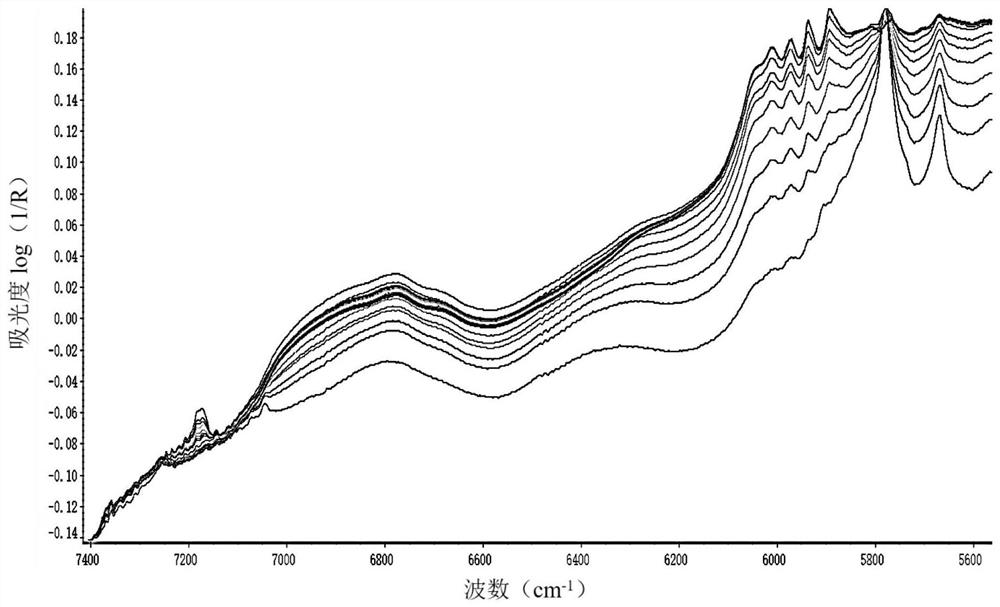
The invention discloses a method for detecting mixing uniformity of hydroxychloroquine sulfate particles. The method comprises the following steps of: acquiring a near infrared spectrum of a mixture in a mixing process, wherein the mixture comprises hydroxychloroquine sulfate particles and magnesium stearate; and calculating a standard deviation value of a collected near infrared spectrum peak area by adopting a moving window standard deviation method. The method for detecting the mixing uniformity of the hydroxychloroquine sulfate particles, disclosed by the invention, is relatively high in accuracy and safety coefficient; and when the detection method is applied to industrial production, the mixing uniformity of the hydroxychloroquine sulfate particles can be rapidly and accurately detected, and the end point of the mixing time can be efficiently and conveniently monitored.
Owner:SHANGAI PHARMA GRP CO LTD +2
Hydroxychloroquine sulfate hydrate as well as crystal form, preparation method and application thereof
PendingCN113603642AImprove moisture absorption stabilityEasy to operateOrganic active ingredientsAntipyreticPhysical chemistryHydroxychloroquine Sulfate
The invention discloses a hydroxychloroquine sulfate hydrate as well as a crystal form, a preparation method and application thereof. The hydroxychloroquine sulfate hydrate, especially the crystal forms B, C and D of the hydrate, has better moisture absorption stability, is easy for industrial production and subsequent preparation operation, is stable and reliable in quality, and has better patent medicine prospects.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
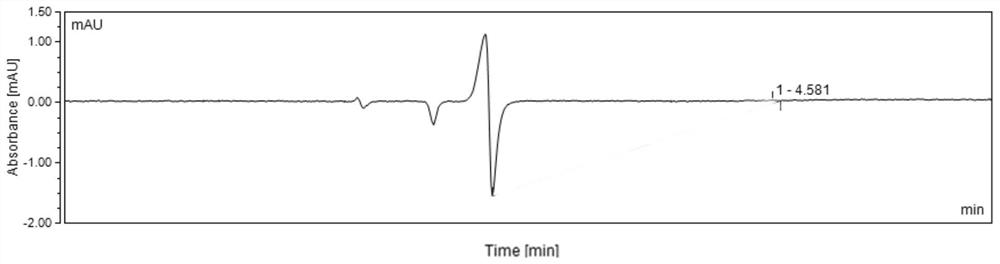
Method for detecting content of hydroxychloroquine nitrogen oxide impurities in hydroxychloroquine sulfate
PendingCN112394112AReliable detection methodEasy to separateComponent separationHydroxychloroquinePhosphate
The invention relates to a method for detecting the content of hydroxychloroquine nitrogen oxide impurities in hydroxychloroquine sulfate. The method comprises the following steps: performing detecting by using liquid chromatography under the following detection conditions: a chromatographic column is a phenyl chromatographic column, the mobile phase is composed of 0.05 mol / L of potassium dihydrogen phosphate solution and methanol, the 0.05 mol / L of potassium dihydrogen phosphate solution is used as a mobile phase A, and the methanol is used as a mobile phase B, and the volume ratio of the mobile phase A to the mobile phase B is 80: 20. The method has a high degree of separation for hydroxychloroquine nitrogen oxide.
Owner:GUANGDONG NEWSOUTH ARTEPHARM CO LTD +1
Method for determining organic impurities in hydroxychloroquine sulfate
InactiveCN111474269AQuality is easy to controlQuality improvementComponent separationSilanesHydroxychloroquine Sulfate
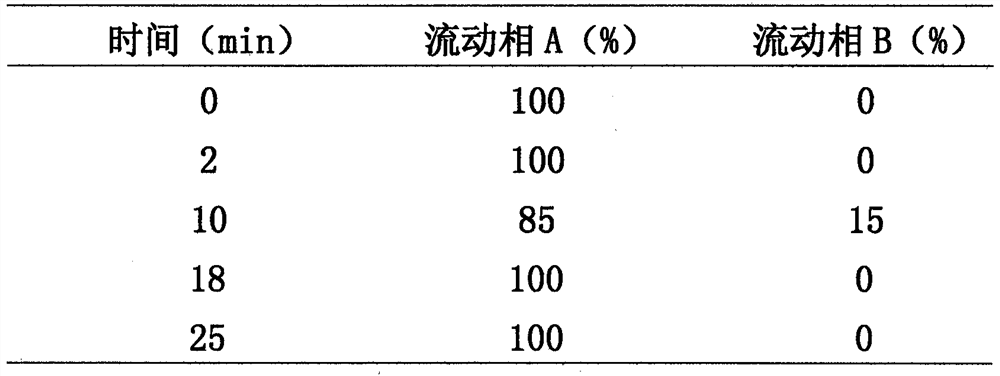
The invention discloses a method for determining organic impurities in hydroxychloroquine sulfate. According to the method, a chromatographic column taking octadecylsilane chemically bonded silica asa filler is adopted, a diode array detector or an ultraviolet detector is used as a detector, the mobile phase is subjected to gradient elution by a mobile phase A (NH3-NH4 < + > buffer solution) anda mobile phase B (ammonia water-acetonitrile mixed solution), the elution procedure (the volume ratio of A to B at different times) is as follows: 0 min (65: 35), 15 min (65: 35), 16 min (35: 65), 30min (35: 65), 30.01 min (65: 35) and 40 min (65: 35). The method is high in specificity and can be used for rapidly detecting the organic impurities in the hydroxychloroquine sulfate.
Owner:CHANGSHA RUHONG MEDICINE TECH CO LTD +1
Hydroxychloroquine sulfate pharmaceutical composition and preparation method thereof
ActiveCN114224890AGood compressibilityGood reproducibilityOrganic active ingredientsImmunological disordersHydroxychloroquine SulfateDrug activity
The invention provides a hydroxychloroquine sulfate pharmaceutical composition and a preparation method thereof. The hydroxychloroquine sulfate pharmaceutical composition comprises the following components in percentage by weight: 60-70% of a pharmaceutical active component, 20-30% of a filler, 2-10% of an adhesive, 2-10% of a disintegrating agent, 0.5-2% of a lubricant and 2-6% of a coating material. The pharmaceutical composition and the preparation method have the advantages that the hygroscopicity is low, the compressibility and the reproducibility are good, the problem of tablet sticking caused by moisture absorption of hydroxychloroquine sulfate particles can be solved, and the production convenience and the stability are greatly improved.
Owner:JIANGSU SEMPOLL PHARMA
Preparation method of hydroxychloroquine sulfate
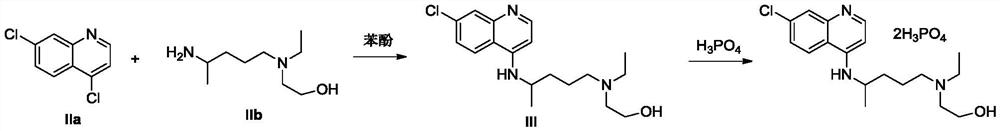
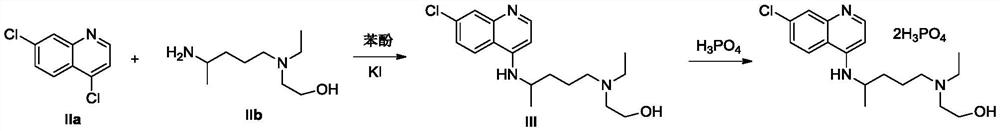
The invention provides a preparation method of hydroxychloroquine sulfate, specifically a preparation method of hydroxychloroquine sulfate. The method comprises the following steps: (1) preparation of hydroxychloroquine: (1.1) in a first solvent, in the presence of KI and an antioxidant, reacting 4, 7-dichloroquinoline (formula I) with an amino side chain as shown in a formula II to obtain a reaction mixture containing hydroxychloroquine (formula III); and (1.2) separating the hydroxychloroquine from the reaction mixture containing the hydroxychloroquine; and (2) preparation of hydroxychloroquine sulfate: (2.1) in a second inert solvent, reacting the hydroxychloroquine obtained in the step (1) with sulfuric acid to form a salt so as to obtain the hydroxychloroquine sulfate (formula IV).
Owner:CINKATE PHARMA INTERMEDIATES
S-hydroxychloroquine sulfate isomer inspection method
InactiveCN112505198AResolve detectionThe detection method is convenient and fastComponent separationDrug utilisationPharmaceutical drug
The invention belongs to the technical field of pharmaceutical analysis, particularly relates to a detection method of a S-hydroxychloroquine sulfate isomer, and provides a convenient, efficient and accurate detection method in order to solve the detection problem of the S-hydroxychloroquine sulfate isomer, and the method can detect the content of the S-hydroxychloroquine sulfate isomer, so that the medication safety is effectively guaranteed and the quality control of hydroxychloroquine sulfate bulk drug is facilitated. The method is convenient, efficient and accurate, completely conforms tothe guidance principle verified by the Chinese pharmacopoeia method in the aspects of system applicability, repeatability, specificity and accuracy, and can be used for quality control of the hydroxychloroquine sulfate bulk drug.
Owner:珠海润都制药股份有限公司
Method for detecting content of hydroxychloroquine sulphate impurities in hydroxychloroquine sulfate
InactiveCN112394110AReliable detection methodEasy to separateComponent separationFluid phasePhosphate
The invention relates to a method for detecting the content of hydroxychloroquine sulphate impurities in hydroxychloroquine sulfate. The method comprises the following steps: performing detecting by using a liquid chromatography under the following detection conditions: a chromatographic column is a phenyl chromatographic column, the mobile phase is composed of 0.05 mol / L of potassium dihydrogen phosphate solution and methanol, the 0.05 mol / L of potassium dihydrogen phosphate solution is used as a mobile phase A, and the methanol is used as a mobile phase B, and the volume ratio of the mobilephase A to the mobile phase B is 80: 20. The method has a high degree of separation for hydroxychloroquine sulphate.
Owner:GUANGDONG NEWSOUTH ARTEPHARM CO LTD +1
Method for detecting content of hydroxychloroquine sulfate
PendingCN112129874AImprove applicabilityEasy to operateComponent separationDipotassium phosphatePhosphoric acid
The invention relates to a method for detecting hydroxychloroquine sulfate. The method comprises the following steps of 1) preparation of a test sample solution, specifically, taking a hydroxychloroquine sulfate test sample, adding an acetonitrile-water mixed solution, dissolving and diluting; 2) preparation of a reference substance solution, specifically, dissolving a hydroxychloroquine sulfate reference substance in the acetonitrile-water mixed solution, and diluting; and 3) a determination method, specifically, measuring the reference substance solution and the test solution, injecting intoa high performance liquid chromatograph, recording a spectrogram, and calculating the content of the test hydroxychloroquine sulfate according to the peak area, wherein the chromatographic conditionsof the high performance liquid chromatography are as follows that octadecylsilane chemically bonded silica is used as a filler, a mobile phase is a mixed solution of a dipotassium phosphate solutionand acetonitrile in a volume ratio of 10 to 90-70 to 30, the flow rate is 0.8-1.2 ml / min, the detection wavelength is 244-264 nm, and the sample size is 5-50mu l. The method has the advantages of goodin the obtained component spike and resolution, simple in operation, high in precision, high in the recovery rate and good in the stability and repeatability.
Owner:JIANGXI GUOYAO PHARMA LLC
New Application of Chloroquine in Controlling Root Knot Nematode
Owner:YUNNAN UNIV
Method for detecting content of hydroxychloroquine sulfate in hydroxychloroquine sulfate tablet
PendingCN114720574ANo need for labor protectionThe detection method is efficient and fastComponent separationFluid phaseOrganic solvent
The invention relates to the technical field of drug analysis and detection, and provides a method for detecting the content of hydroxychloroquine sulfate in hydroxychloroquine sulfate tablets, and the method comprises the following steps: preparing a sample analysis solution from the hydroxychloroquine sulfate tablets; and analyzing the sample analysis solution by using a high performance liquid chromatography to determine the content of hydroxychloroquine sulfate in the hydroxychloroquine sulfate tablet. Wherein in the step of liquid chromatography, a used mobile phase is a mixed solvent composed of a buffer solution and an organic solvent, the buffer solution is an ammonium formate solution, the organic solvent is acetonitrile, and the volume ratio range of ammonium formate to acetonitrile in the mixed solvent is (80-87): (13-20). The invention provides a method for efficiently detecting the content of hydroxychloroquine sulfate in hydroxychloroquine sulfate tablets without solvent poison prevention labor protection.
Owner:GRAND PHARM (CHINA) CO LTD
Method for detecting content of hydroxychloroquine sulfate in artemisinin hydroxychloroquine sulfate tablets
PendingCN112394111AShort equilibration timeFast measurementComponent separationArtemisininsPhosphate
The invention relates to a method for detecting the content of hydroxychloroquine sulfate in artemisinin hydroxychloroquine sulfate. The method comprises the following steps: performing detecting by using a liquid chromatography under the following detection conditions: an octadecylsilane chemically bonded silica chromatographic column is used as a chromatographic column, the mobile phase is composed of a potassium dihydrogen phosphate solution and methanol, the potassium dihydrogen phosphate solution is used as a mobile phase A, and the methanol is used as a mobile phase B, the volume ratio of the mobile phase A to the mobile phase B is (1-25): (75-99). The method has a good separation degree on hydroxychloroquine sulfate peaks.
Owner:GUANGDONG NEWSOUTH ARTEPHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com