Method for detecting content of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and a detection method, which is applied in the field of medicine, can solve problems such as insufficient sensitivity and complex process, and achieve the effects of good stability and repeatability, simple operation, and good system applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
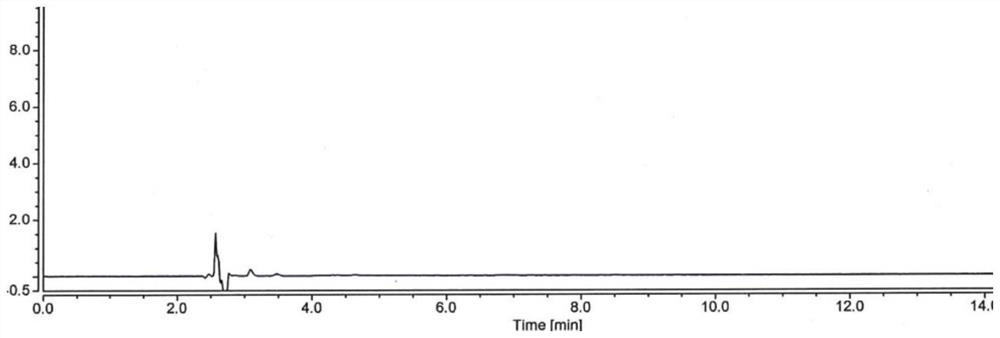
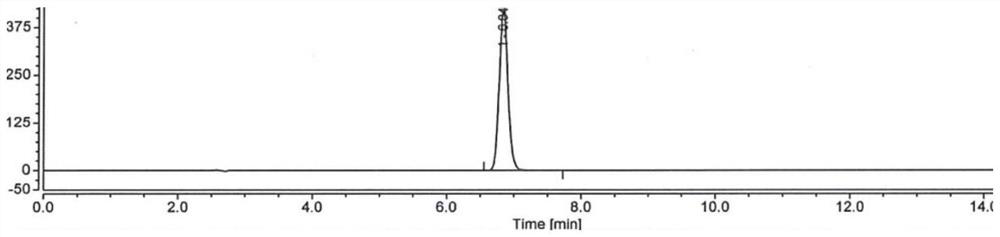
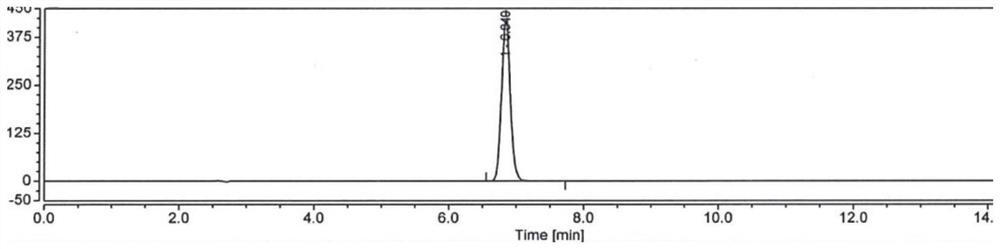
[0049] Embodiment 1: use the method for high performance liquid chromatography to detect hydroxychloroquine sulfate content
[0050] Take 1L of purified water, add 0.02mol of dipotassium hydrogen phosphate (3.48g), dissolve and add phosphoric acid to adjust the pH to 6.0, take 350ml of the above solution, add 650ml of acetonitrile, mix well, and filter to obtain the mobile phase;
[0051] Take 500ml of acetonitrile, add 500ml of purified water, mix well, and prepare a diluted solution;
[0052] Take about 20 mg of hydroxychloroquine sulfate, accurately weighed, put in a 100ml measuring bottle, add acetonitrile water (1:1) to dissolve and dilute to the mark, shake well, accurately measure 5ml and put it in a 50ml measuring bottle, add acetonitrile water (1:1) 1) Dilute to the mark and shake well to prepare the test solution;
[0053]Take about 20mg of hydroxychloroquine sulfate reference substance, accurately weighed, put in a 100ml measuring bottle, add acetonitrile water (1:...
Embodiment 2
[0056] Embodiment 2: Dipotassium hydrogen phosphate solution and acetonitrile ratio investigation
[0057] Three mobile phases with volume ratios of 20:80, 35:65, and 50:50 were prepared using the 0.02 mol / L dipotassium hydrogen phosphate solution and acetonitrile prepared according to Example 1. The rest of the conditions are the same as in Example 1, and the resolution, main peak retention time and impurity peak quantity of different mobile phases are shown in Table 1.
[0058] Table 1 The resolution, main peak retention time and impurity peak quantity of different mobile phase ratios
[0059] Dipotassium hydrogen phosphate: acetonitrile Main peak retention time (min) Separation Number of impurity peaks 20:80 4.561 1.632 4 35:65 6.840 2.331 4 50:50 8.353 3.152 3
[0060] It can be seen that with the decrease of the organic phase in the mobile phase, the retention time of the main peak is prolonged, the separation between the main pea...
Embodiment 3
[0065] Embodiment 3: the investigation to dipotassium hydrogen phosphate solution concentration
[0066] Take 1L of purified water, add 0.01mol (1.74g), 0.02mol (3.48g) and 0.03mol (5.22g) of dipotassium hydrogen phosphate, dissolve and add phosphoric acid to adjust the pH to 6.0, take 350ml of the above solution, mix it with 650ml of acetonitrile, Three mobile phases were prepared separately. All the other conditions are the same as in Example 1. The resolution, retention time of main peak and number of impurity peaks of different mobile phases are shown in Table 2.
[0067] The degree of separation and main peak retention time and impurity peak quantity of different hydroxychloroquine sulfate solution concentration of table 2
[0068] Dipotassium hydrogen phosphate solution concentration (mol / L) Main peak retention time (min) Separation Number of impurity peaks 0.01 6.713 2.282 4 0.02 6.820 2.331 4 0.03 6.991 2.410 4
[0069] With ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com