Hydroxychloroquine sulphate solid preparation and preparation method thereof
A technology of hydroxychloroquine sulfate and solid preparation, applied in the field of hydroxychloroquine sulfate solid preparation and preparation thereof, to achieve the effects of improving stability, excellent dissolution and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
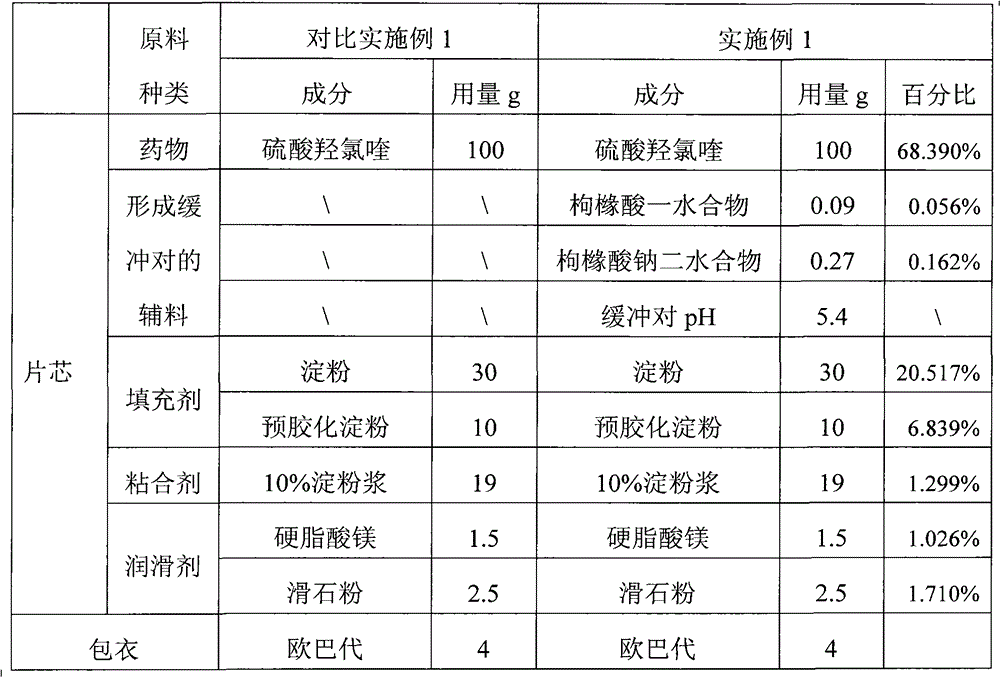
Examples
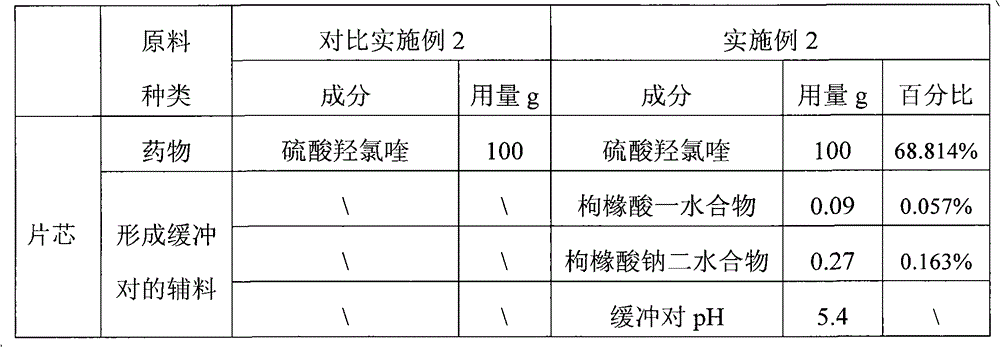
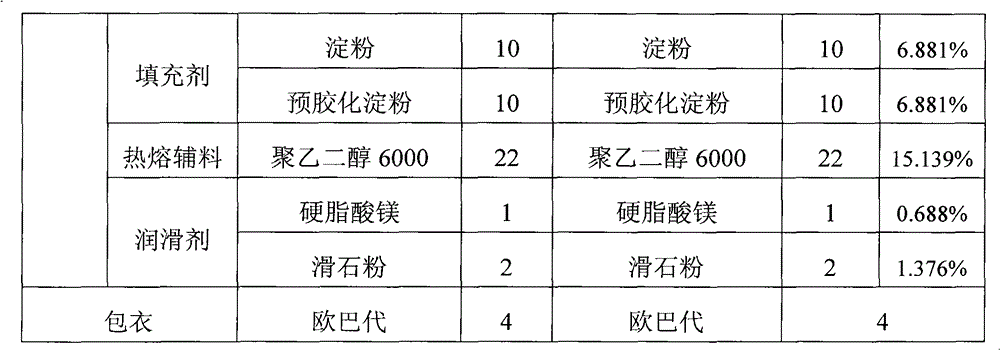
Embodiment 2
[0042] Example 2: Dissolve citric acid monohydrate and sodium citrate dihydrate in 10 times the mass of water, add starch and mix evenly, pass through a 24-mesh sieve twice, pass through a 80-mesh sieve after drying, and set aside. After the hydroxychloroquine sulfate is pulverized by a pulverizer, it is evenly mixed with the master powder of the buffering auxiliary material, pregelatinized starch and powdery polyethylene glycol 6000.
[0043] (2) Granulation and coating
[0044] Put it in a fast mixer with a jacket temperature of 70-85°C, turn on the agitator of the granulator, discharge when the material temperature reaches 61°C, cool down, and granulate with a 20-mesh sieve. Add magnesium stearate and talcum powder, mix evenly, and compress into tablets to obtain tablet cores. Add Opadry powder while stirring in water, continue to stir for 45 minutes after adding, be made into the coating solution of 19wt% concentration, carry out film coating to tablet core.
Embodiment 3
[0045] Embodiment 3 Hydroxychloroquine sulfate sheet (100mg / sheet)
[0046]
[0047]
[0048] Preparation process: Dissolve disodium hydrogen phosphate and citric acid in water of 10 times the mass, add microcrystalline cellulose and mix evenly, pass through a 24-mesh sieve twice, pass through a 80-mesh sieve after drying, and set aside. Hydroxychloroquine sulfate is pulverized by a pulverizer, mixed evenly with buffered auxiliary material powder, pregelatinized starch and powdered polyethylene glycol 6000, placed in a fast mixer with a jacket temperature of 70-85°C, and the granulator agitator is turned on , when the temperature of the material reaches 63°C, the material is discharged, cooled, and granulated with a 20-mesh sieve. Add magnesium stearate and talcum powder, mix evenly, and compress into tablets to obtain tablet cores. Add Opadry powder while stirring in water, continue to stir for 45 minutes after adding, be made into the coating solution of 19wt% concen...
Embodiment 4
[0049] Embodiment 4 hydroxychloroquine sulfate capsules (100mg / grain)
[0050] Get the granules before tablet compression of Example 3, mix evenly after passing through a 30-mesh sieve, and pack into capsules.
[0051] Embodiment 5 and 6 hydroxychloroquine sulfate sheet (100mg / sheet)
[0052]
[0053]
[0054] Preparation process: disperse hypromellose in hot water at 80°C, add water and stir to dissolve, make hypromellose aqueous solution, add 5% hydrochloric acid, citric acid and sodium hydroxide, stir and dissolve, and prepare buffered Granulating liquid. Hydroxychloroquine sulfate is pulverized by a pulverizer, mixed evenly with starch and pregelatinized starch, added to the above-mentioned granulation solution to stir and granulate, and the wet granules are dried and sized. Add magnesium stearate and talcum powder, mix evenly, and compress into tablets to obtain tablet cores. Add Opadry powder while stirring in water, continue to stir for 45 minutes after addin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com