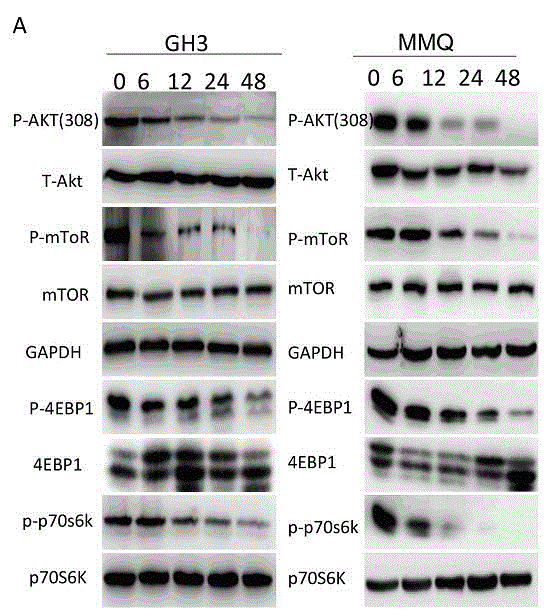
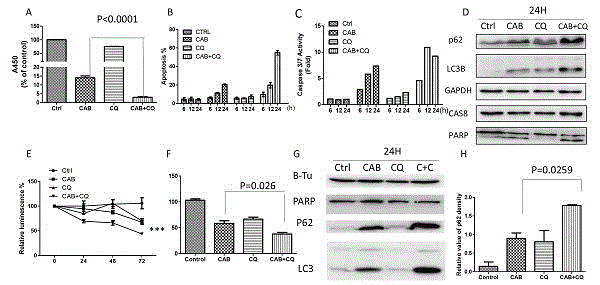
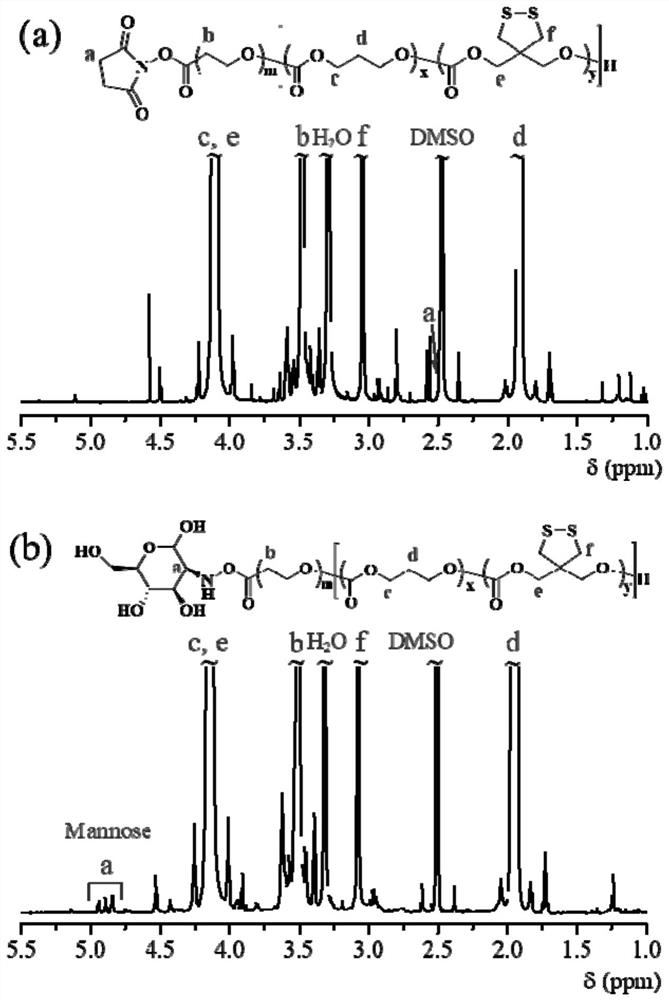
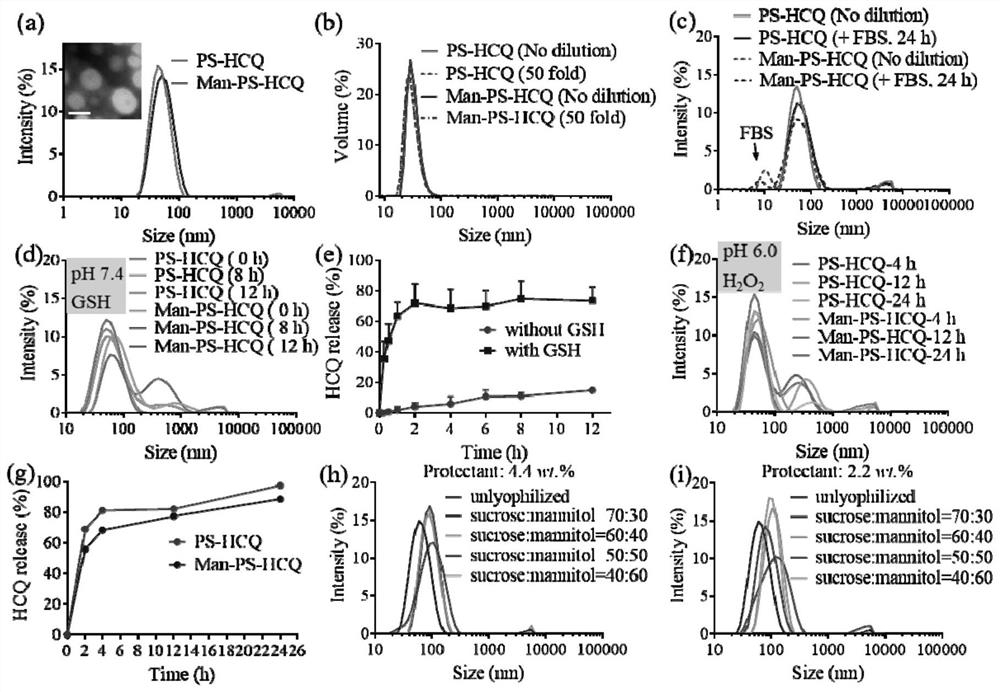
Patents
Literature
117 results about "Chloroquina" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
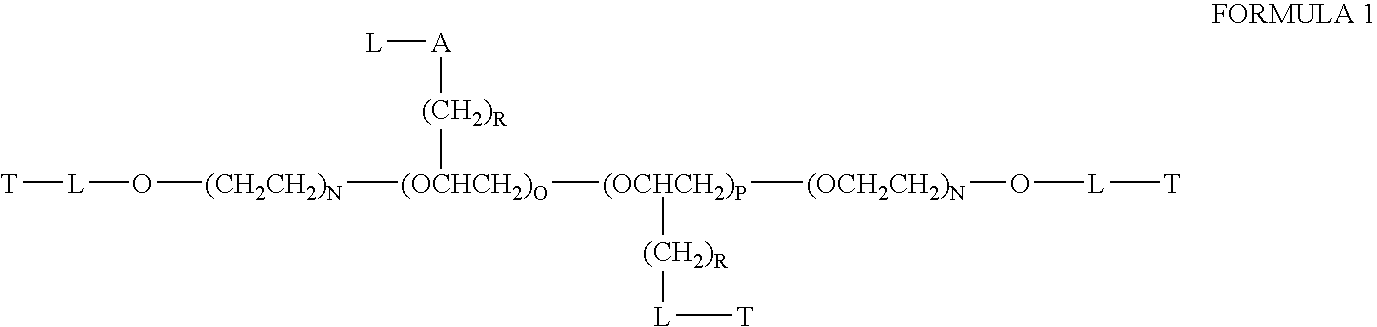
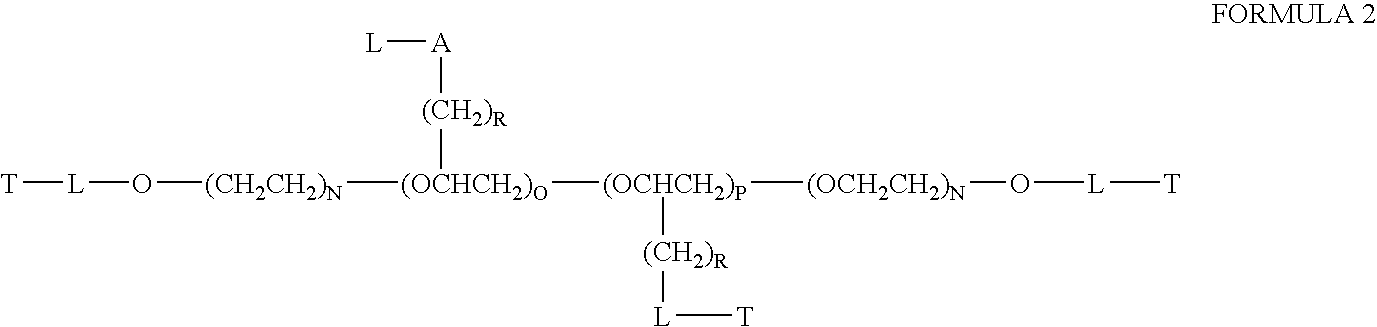
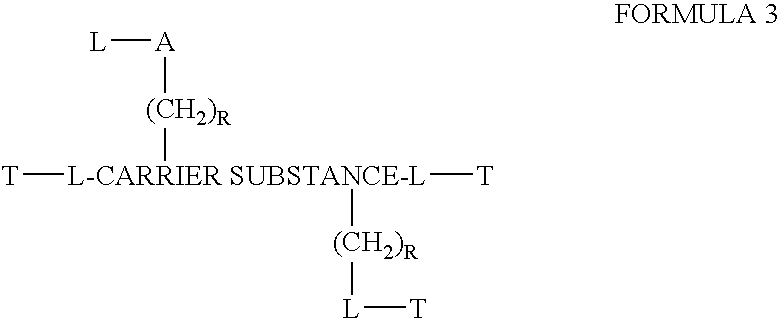
Chloroquine coupled nucleic acids and methods for their synthesis
InactiveUS20060040879A1Overcome limitationsFacilitated releaseSugar derivativesGenetic therapy composition manufactureTherapeutic effectPharmaceutical Substances
This invention discloses compositions and methods for preparing chloroquine-coupled nucleic acid compositions. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the nucleic acid needs to be released, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a nucleic acid directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing nucleic acids for therapeutic or other medical uses. The invention also discloses nucleic acid carrier compositions that are coupled to targeting molecules for targeting the delivery of nucleic acids to their site of action.
Owner:KOSAK KENNETH
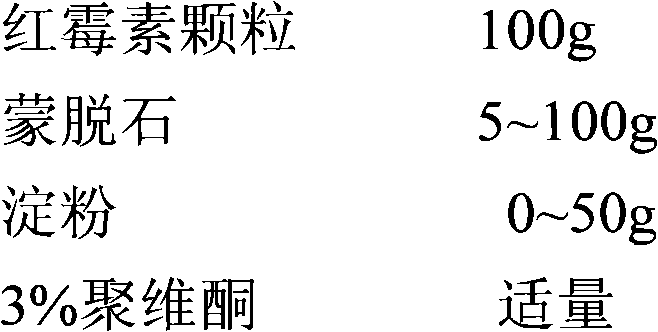
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
Remdesivir treatment methods
PendingUS20210393653A1Reduce riskOrganic active ingredientsAntiviralsPharmaceutical medicineViral infection
Provided herein are methods of treating or preventing a viral infection in a subject comprising administering a compound of Formula I, Formula Ia, or Formula Ib, or a pharmaceutically acceptable salt thereof, wherein the subject is not being treated with chloroquine, or an analog or salt thereof.
Owner:GILEAD SCI INC
Hydroxychloroquine sulphate solid preparation and preparation method thereof
ActiveCN102525969BGood dissolution effectImprove stabilityOrganic active ingredientsAntipyreticBuffer solutionMedicinal chemistry
Owner:SHANGHAI ZHONGXI PHARMA
Genetic alteration associated with pre-malignant cancer
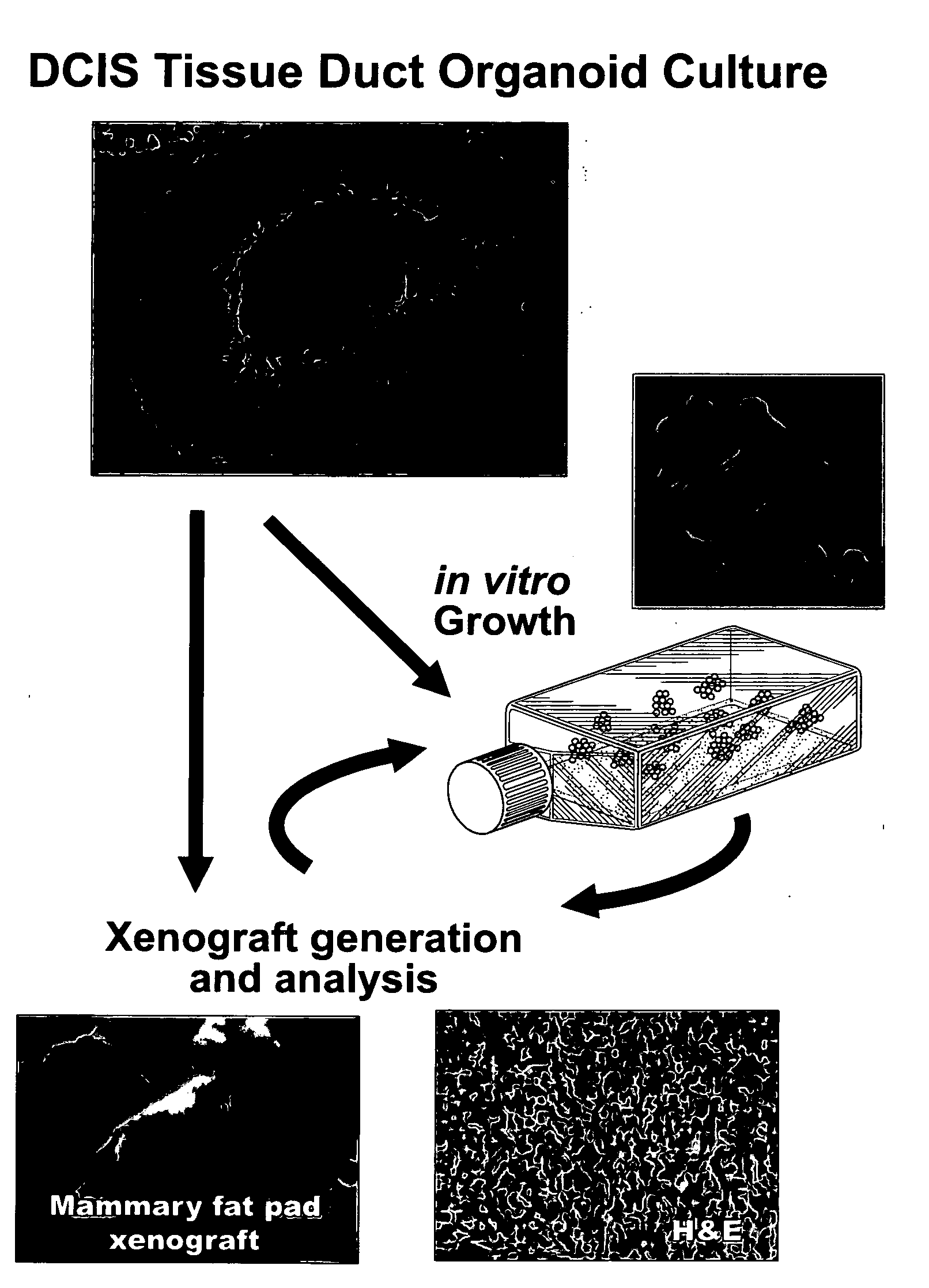
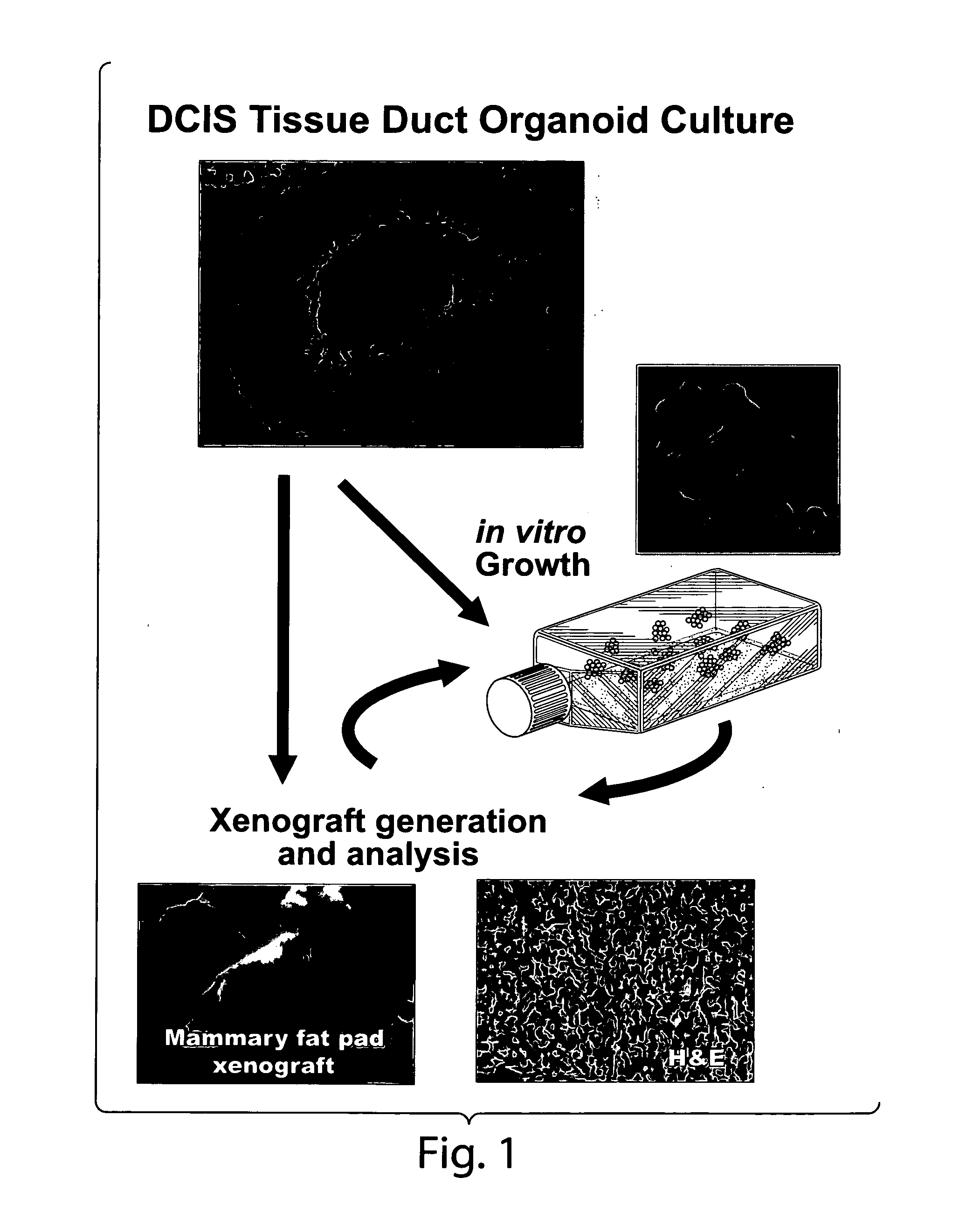
Described herein are progenitor cancer cells and cell lines isolated from human breast ductal carcinoma in situ (DCIS) lesions and the uses of these cells or cell lines in drug design, drug screening, and monitoring in vivo therapy. The DCIS malignant precursor cells or cell lines are epithelial in origin, are positive for markers of autophagy, show at least one genetic difference from normal cells of said fragment, form 3-D tube-like structures or ball aggregates, or are inhibited in formation of 3-D structures and migration by treatment with chloroquine. In one embodiment, there is a loss of heterozygosity (LOH) that is narrowly confined to a region of chromosome 6p (6p21.1-6p12.3) that contains the SUPT3H gene.
Owner:GEORGE MASON UNIVERSITY
Remdesivir treatment methods
ActiveUS20220175805A1Reduce riskOrganic active ingredientsAntiviralsPharmaceutical medicineViral infection
Provided herein are methods of treating or preventing a viral infection in a subject comprising administering a compound of Formula I, Formula Ia, or Formula Ib, or a pharmaceutically acceptable salt thereof, wherein the subject is not being treated with chloroquine, or an analog or salt thereof.
Owner:GILEAD SCI INC
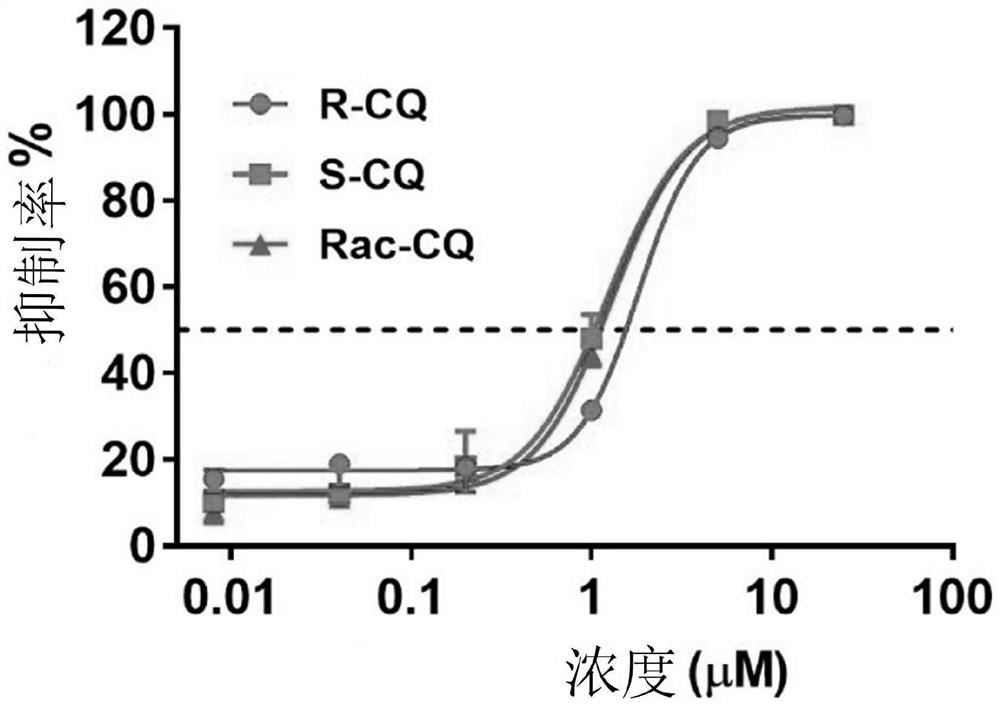
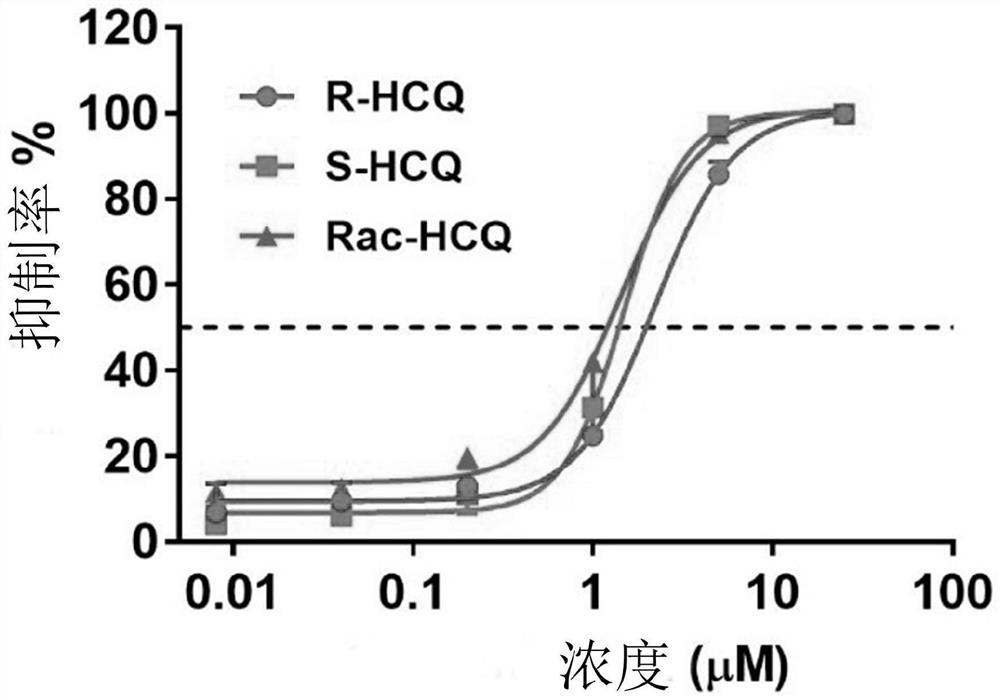
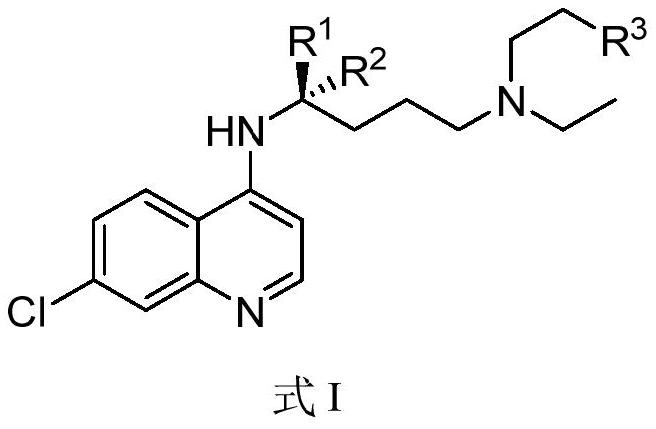
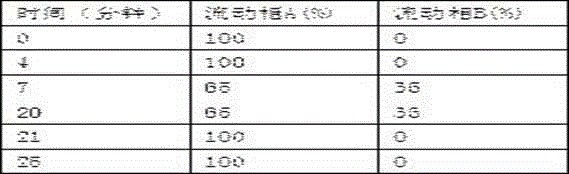
Chiral chloroquine, hydroxychloroquine and derivatives thereof as well as preparation method and application of chiral chloroquine, hydroxychloroquine and derivatives thereof
ActiveCN111620815AEasy to operateSuppress deathOrganic active ingredientsOrganic chemistry methodsHydroxychloroquineFluid phase
The invention belongs to the field of drug synthesis, and discloses a compound with a structure as shown in a formula I (See the specification) and pharmaceutically acceptable salt, tautomer, polymorphic substance, isomer and solvate thereof. Secondly, the invention discloses a method for preparing chiral chloroquine and hydroxychloroquine through chiral high performance liquid chromatography. Finally, the invention discloses application of chiral chloroquine, hydroxychloroquine and salt derivatives thereof in preparation of drugs for treating novel coronavirus pneumonia.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Compound reserpine medicine composition for treating hypertension and preparation method of compound reserpine medicine composition
InactiveCN105193841AImprove stabilityImprove standardsPill deliveryPharmaceutical non-active ingredientsVitamin b6Efficacy
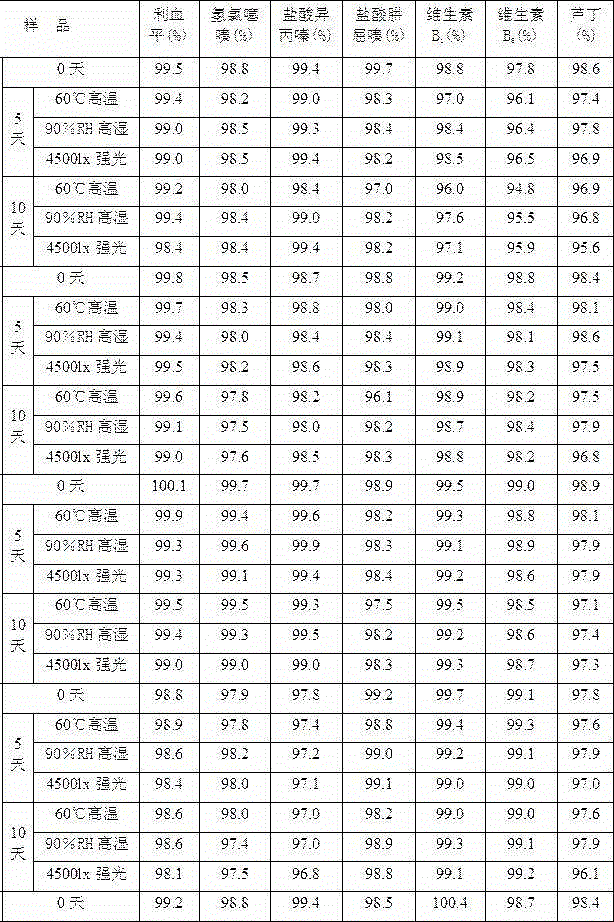
The invention belongs to the field of pharmacy, and particularly relates to a compound reserpine medicine composition for treating hypertension and a preparation method of the compound reserpine medicine composition. The compound reserpine medicine composition comprises raw materials in parts by weight as follows: 0.03 parts of reserpine, 1 part of hydralazine hydrochloride, 0.025 parts of cyclopenthiazide, 1.5 parts of hydrochlorothiazide, 2 parts of promethazine hydrochloride, 30 parts of potassium chloride, 5 parts of rutin, 2.5 parts of chloroquine phosphate, 1 part of vitamin B1, 1 part of vitamin B6, 2-10 parts of a stabilizer fumaric acid and other pharmaceutical adjuvants. Experiment results show that the medicine composition takes effect quickly, has long-lasting medicine efficacy, facilitates long-term treatment of a patient and is safe to take; besides, the formula has a high technological stability and is not influenced by environmental temperature and humidity, differences between different bathes of preparations are remarkably reduced, and the stability of samples is improved.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Irinotecan and chloroquine pharmaceutical composition and common carrier liposome and preparation thereof
ActiveCN104971062AImprove convenienceSimple preparation processOrganic active ingredientsAntineoplastic agentsCholesterolPhospholipid
The invention discloses an irinotecan and chloroquine pharmaceutical composition, and a common carrier liposome and preparation thereof. The irinotecan and chloroquine in the pharmaceutical composition are in the weight ratio of 1-100:1-00. The irinotecan and chloroquine pharmaceutical composition common carrier liposome comprises the drug, phospholipid, cholesterol compound and an aqueous phase, wherein the weight ratio of the drug, phospholipid and cholesterol compound is 1:2-100:0.8-35, and the drug is irinotecan drug and chloroquine drug. Synergic effect of irinotecan and chloroquine enhances the killing effect of irinotecan drug on sensitive strain cells of colon cancer. The preparation method of the common carrier liposome uses a pH gradient method or ammonium sulfate gradient method, has the advantages of simple preparation process, few parameter control and lower reaction conditions, and is helpful to reduce production cost and easy for industrial production.
Owner:ZHEJIANG UNIV
Application of polycyclic polyketone compound in preparation of medicine for resisting novel coronavirus
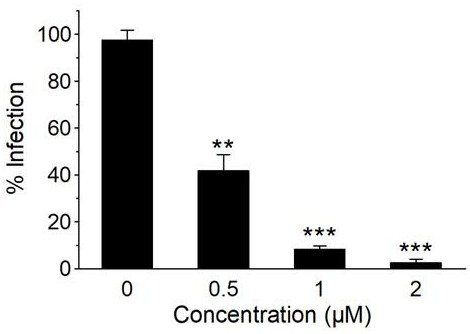
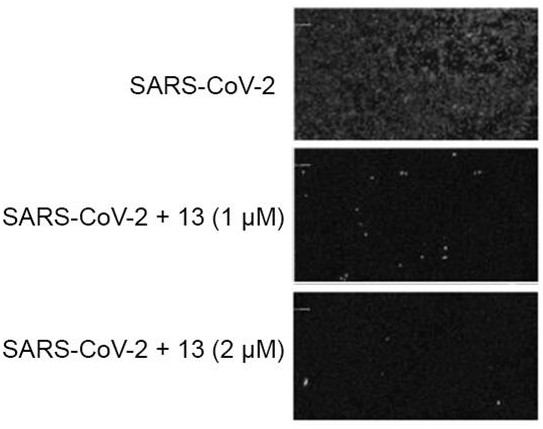
The invention discloses an application of a polycyclic polyketone compound in preparation of a medicine for resisting a novel coronavirus SARS-CoV-2. The polyketone compound disclosed by the invention has an inhibition effect on a novel coronavirus SARS-CoV-2 at a cellular level, can be used for remarkably reducing the virus titer of the virus in cells and inhibiting cytopathy induced by the virus, and has concentration dependence. In addition, the polyketone compound has chemical structure types different from those of the ridecevir and the chloroquine phosphate, and is expected to be developed into a novel medicine for resisting the novel coronavirus SARS-CoV-2. Therefore, the compound has a good application prospect in treatment of related diseases caused by infection of novel coronavirus SARS-CoV-2.
Owner:JINAN UNIVERSITY
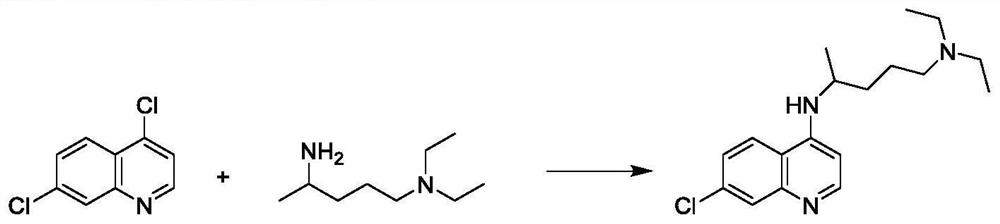
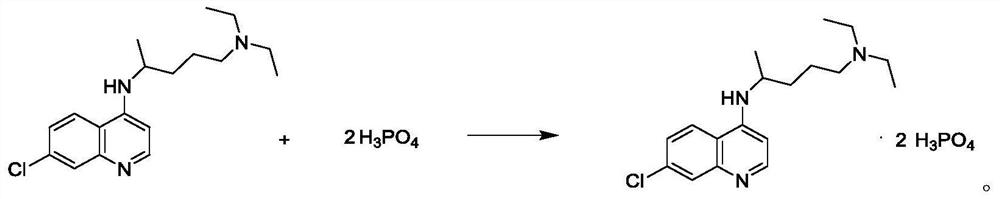
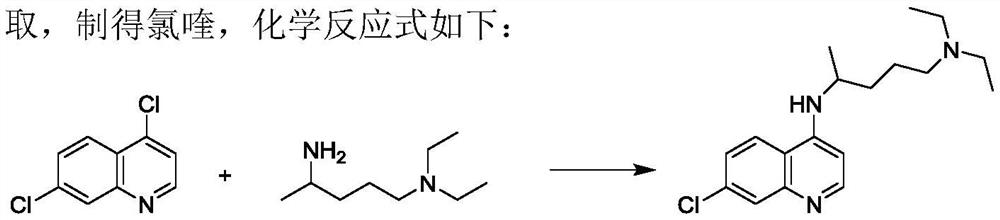
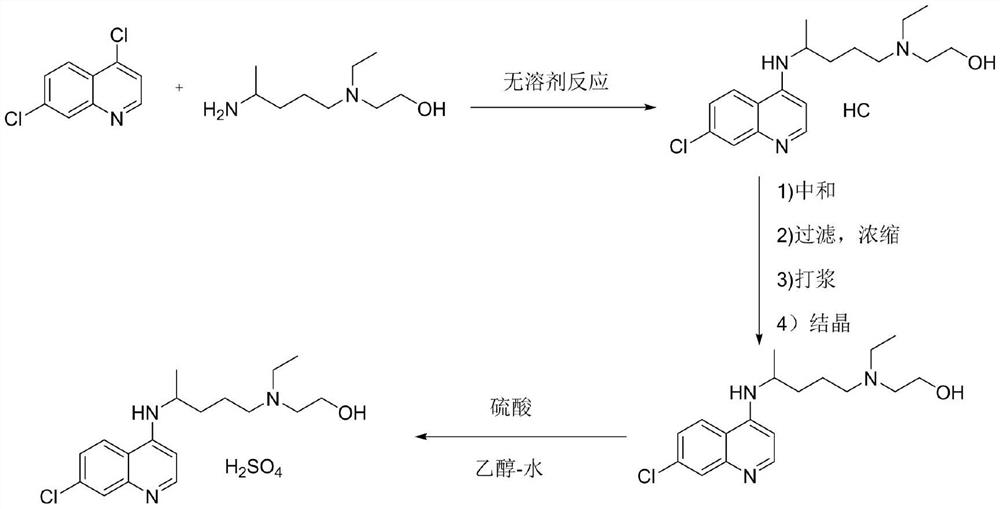
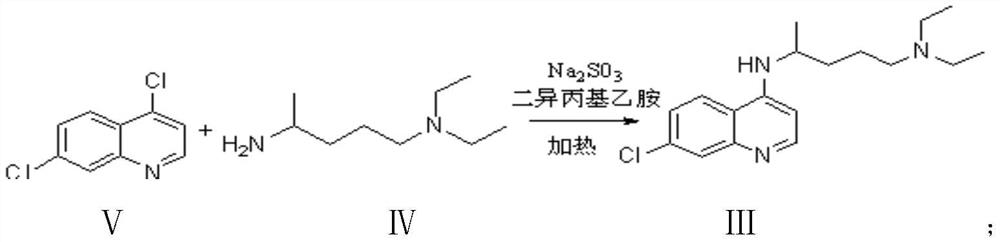
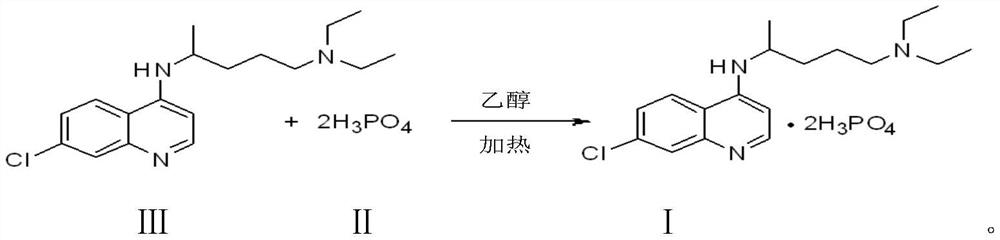
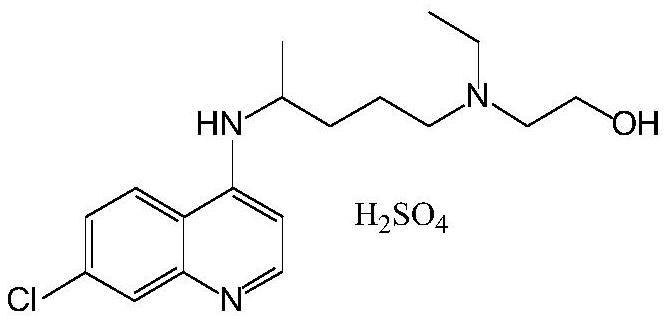
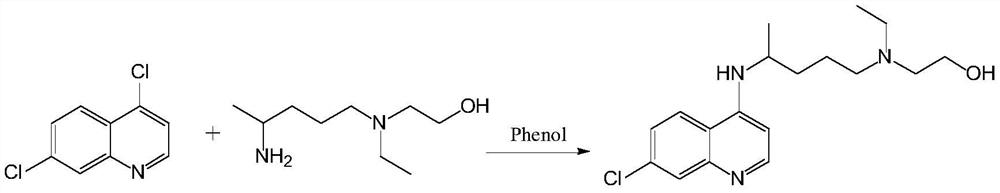
Preparation process of chloroquine phosphate
ActiveCN111662229AReduce usageHigh purityOrganic chemistryAgainst vector-borne diseasesO-Phosphoric AcidPtru catalyst
The invention discloses a preparation process of chloroquine phosphate. The preparation process of the chloroquine phosphate comprises the following steps of: (1) taking 4, 7-dichloroquinoline as an initial raw material, carrying out condensation reaction with 2-amino-5-diethylaminopentane, and carrying out alkalization extraction to obtain chloroquine; and (2) salifying the chloroquine obtained in the step (1) with phosphoric acid to obtain chloroquine phosphate. The invention provides a preparation process of chloroquine phosphate. In the preparation process, the use of organic solvents suchas benzene and other catalysts such as phenol is avoided, and a condensation crystallization impurity removal step is introduced, so that the product purity is high, the individual impurity is less than 0.1%, the green chemical requirements and pharmacopeia standards are met, the operation is simple and convenient, the process is simplified, the production efficiency is improved, and the method is suitable for industrial production.
Owner:JINGHUA PHARMA GRP NANTONG
Crystals of hydroxychloroquine sulfate
PendingCN113527202AOrganic active ingredientsOrganic chemistry methodsHydroxychloroquine SulfateCondensed matter physics
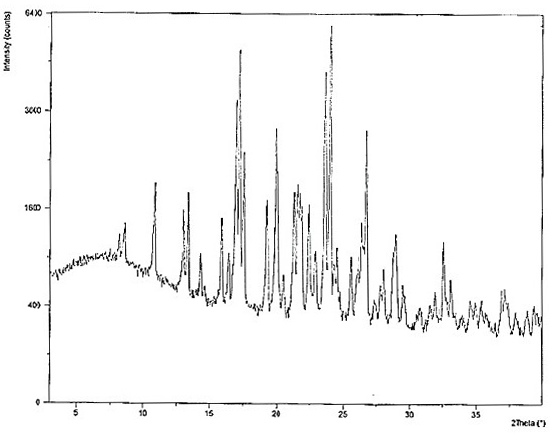
Two crystals of (S)-(+)-hydroxychloroquine sulfate. One crystal features diffraction peaks at 12.3 + / - 0.1 degrees, 13.1 + / - 0.1 degrees, 17.9 + / - 0.1 degrees, 22.8 + 0.1 degrees, 23.4 + 0.1 degrees, 25.1 + / - 0.1 degrees, and 26.3 + / - 0.1 degrees as 2theta angles in a powder X-ray diffraction pattern. The other crystal features diffraction peaks at 12.8 + / - 0.1 degrees, 14.5 + / - 0.1 degrees, 16.7 + 0.1 degrees, 17.6 + 0.1 degrees, 20.2 + / - 0.1 degrees, 21.4 + / - 0.1 degrees, 23.8 + / - 0.1 degrees, 25.7 + / - 0.1 degrees, and 26.0 + / - 0.1 degrees as 2theta angles in a powder X-ray diffraction pattern. Also disclosed are methods of preparing crystals of (S)-(+)-hydroxychloroquine sulfate.
Owner:GENELABS TECH INC
Medicine composition for treating pituitary adenoma, application of medicine composition, medicine box and packaging piece
ActiveCN104922672AEnhanced inhibitory effectImprove clinical efficacyAntineoplastic agentsEndocrine system disorderHydroxychloroquinePharmaceutical drug
The invention belongs to the technical field of biological medicine and relates to a medicine composition for treating pituitary adenoma, application of the medicine composition, a medicine box and a packaging piece. The medicine composition, the medicine box and the packaging piece contain chloroquine / hydroxychloroquine which serves as a combined preparation and is used simultaneously, separately or sequentially, and a therapeutically effective amount of dopamine receptor agonist, and the dopamine receptor agonist is cabergoline or bromocriptine. When the medicine composition, the medicine box and the packaging piece are used for treating tumors, the medicine effect which is superior to the medicine effect obtained by separately using the cabergoline can be achieved, and the clinical treatment effect is improved.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Vesicle nano-drug loaded with chloroquine compound as well as preparation method and application of vesicle nano-drug
ActiveCN113679670AReduce secretionPromote enrichmentOrganic active ingredientsPowder deliveryHydroxychloroquineSide chain
The invention discloses a vesicle nano-drug loaded with a chloroquine compound as well as a preparation method and application of the vesicle nano-drug. The vesicle nano-drug loaded with the chloroquine compound is prepared by taking a polymer and a chloroquine compound as raw materials, wherein the polymer comprises a hydrophilic chain segment and a hydrophobic chain segment; a side chain of the hydrophobic chain segment is dithiolane containing a disulfide bond. According to the vesicle nano-drug loaded with the chloroquine compound disclosed by the invention, a safe and efficient macrophage-targeted nano-drug is researched and developed for treating rheumatoid arthritis; polymer vesicles are designed for efficiently loading, carrying out targeted delivery and controlling to release drug hydroxychloroquine or chloroquine, so that the enrichment of the drug in cytoplasm is improved; M1M is re-polarized to M2M, so that secretion of proinflammatory cytokines is reduced and secretion of anti-inflammatory cytokines is increased; and the drug can be used for inhibiting DC activation and also can be used for removing ROS and enriching in inflammatory joints. In-vitro and in-vivo experiment results prove that the vesicle nano-drug loaded with the chloroquine compound can be used for carrying out targeted treatment on the rheumatoid arthritis.
Owner:SUZHOU UNIV
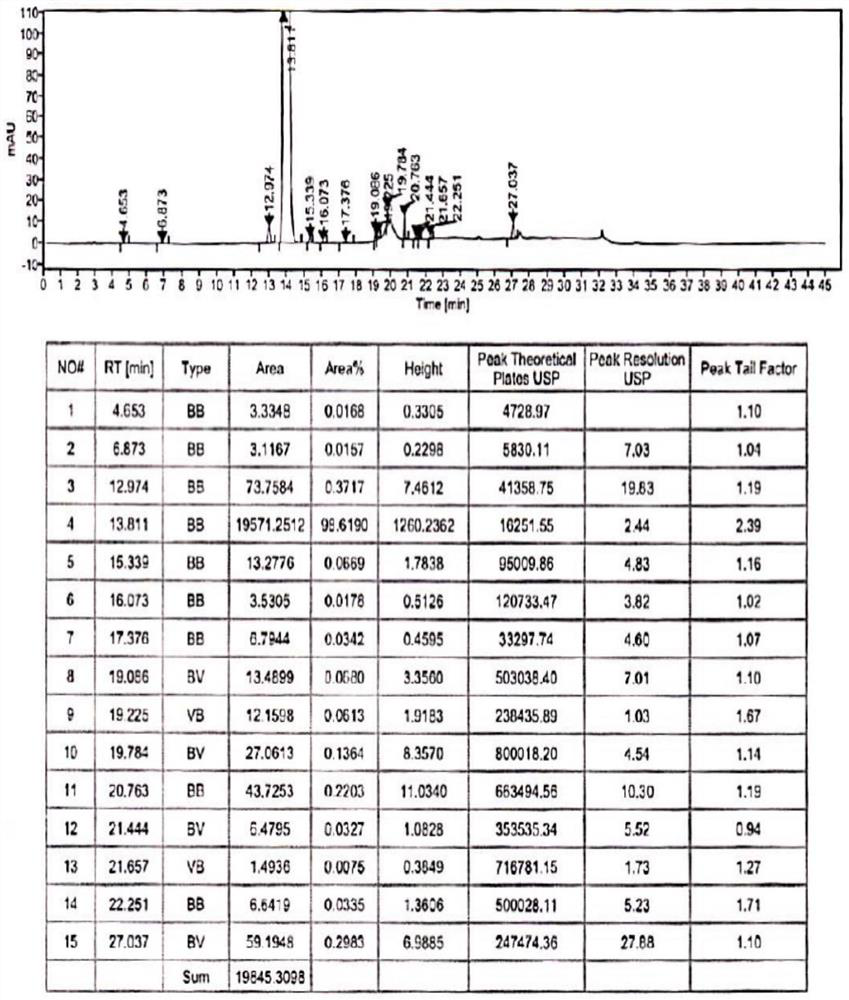
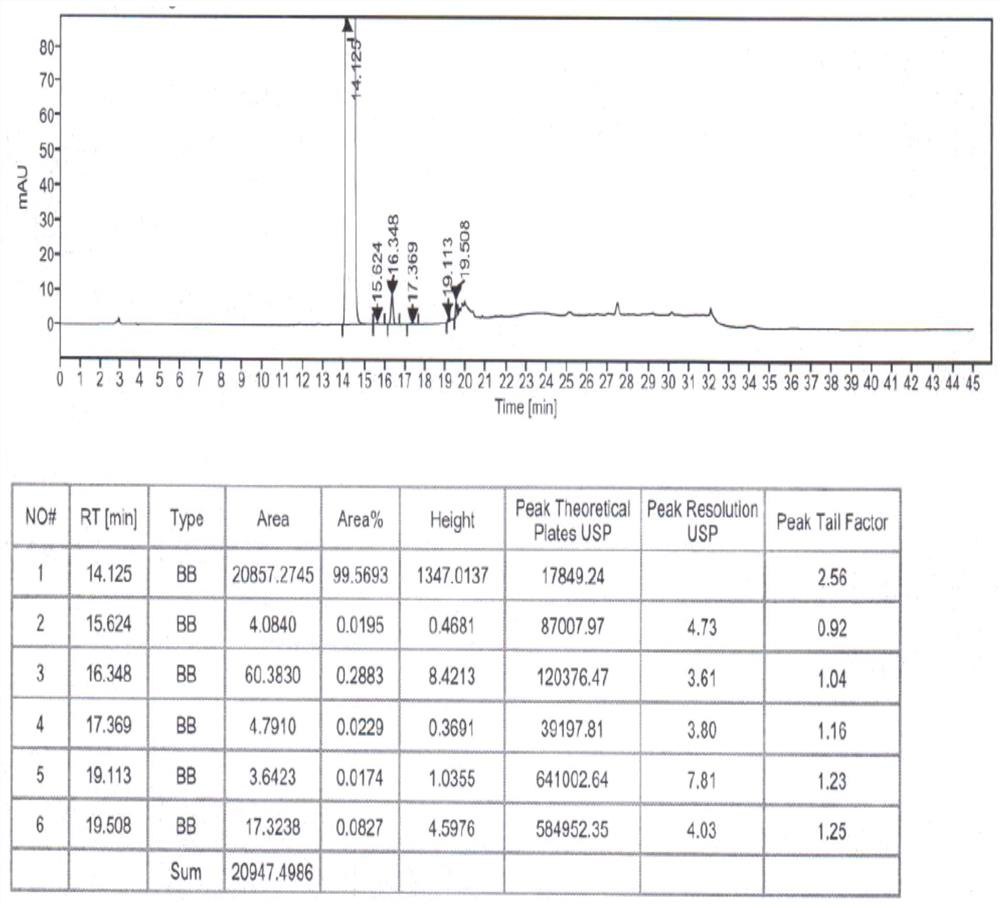
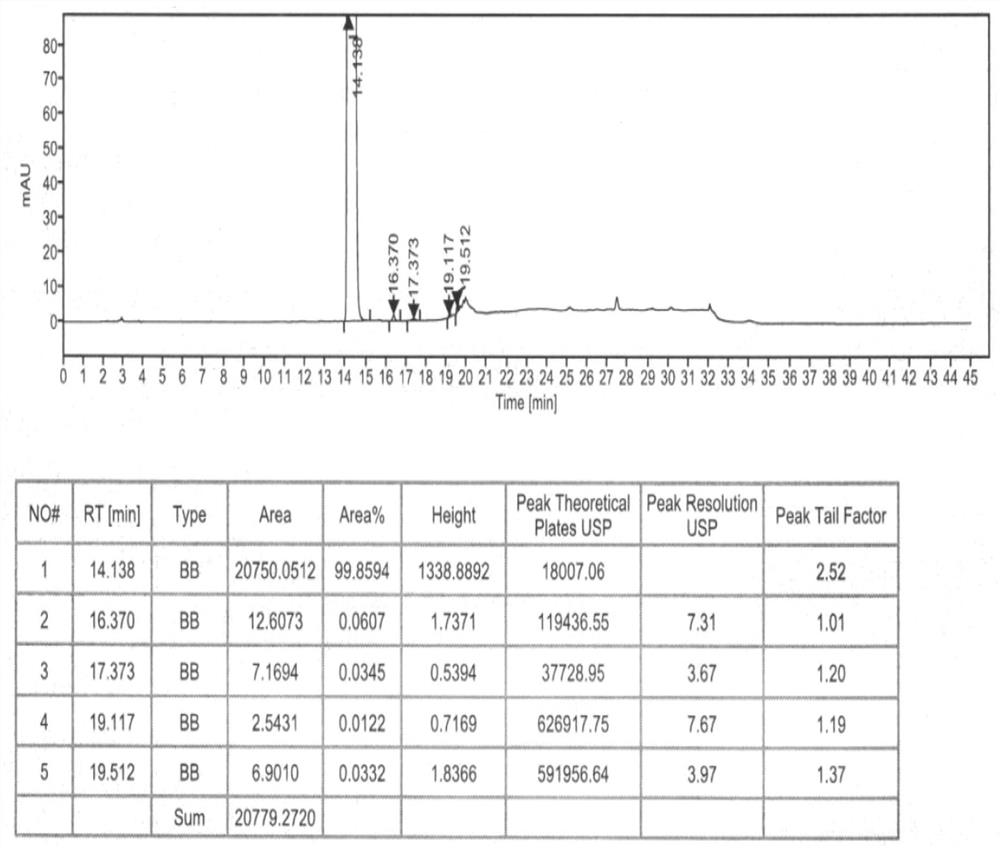
Method for analyzing composition of hydroxychloroquine sulfate preparation by high performance liquid chromatography
PendingCN111398475AImprove accuracyEasy to separateComponent separationHydroxychloroquineHydroxychloroquine Sulfate
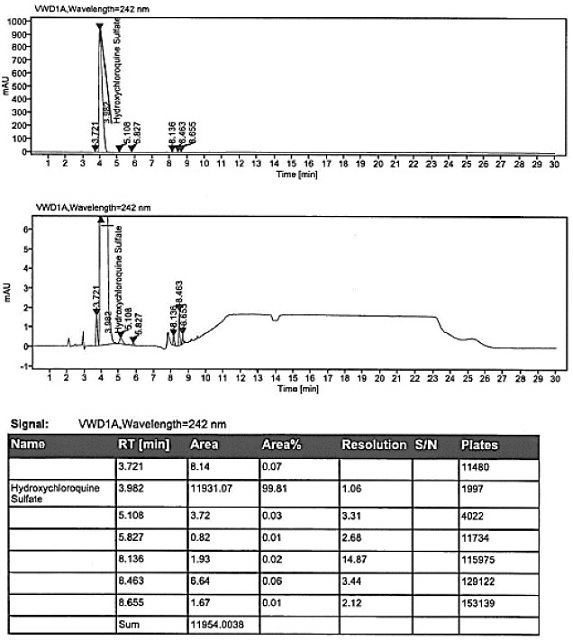
The invention discloses a method for analyzing a composition of a hydroxychloroquine sulfate preparation by high performance liquid chromatography. The method comprises the following steps: a. selecting 50mg of hydroxychloroquine sulfate reference substance, and precisely weighing; b, precisely weighing 1.0 ml, 2.0 ml, 3.0 ml, 4.0 ml, 5.0 ml, 6.0 ml and 7.0 ml of the mixed solutions obtained in the step a, and putting the mixed solutions into a 10ml volumetric flask for later use; c, performing chromatographic analysis by using a chromatographic column to obtain a regression equation of a concentration C and a peak area A; and d, removing a proper amount of a mixture obtained in the step a, precisely weighing, adding a diluent to dissolve, and quantitatively diluting to prepare a solutioncontaining 0.5 mg per 1ml to obtain a test solution; and precisely measuring the test solution, adding a mobile phase, diluting to prepare a solution containing 0.05 g per 1ml as a control solution, precisely measuring 0.2 ml of the control solution, injecting the control solution into a liquid chromatograph, and adjusting sensitivity of an instrument for testing. The method for analyzing the composition of the hydroxychloroquine sulfate preparation by the high performance liquid chromatography is simple and convenient to operate, sensitive, high in accuracy, suitable for clinical hydroxychloroquine serum drug concentration monitoring and high in specificity.
Owner:NANJING MEIRUI PHARMA CO LTD
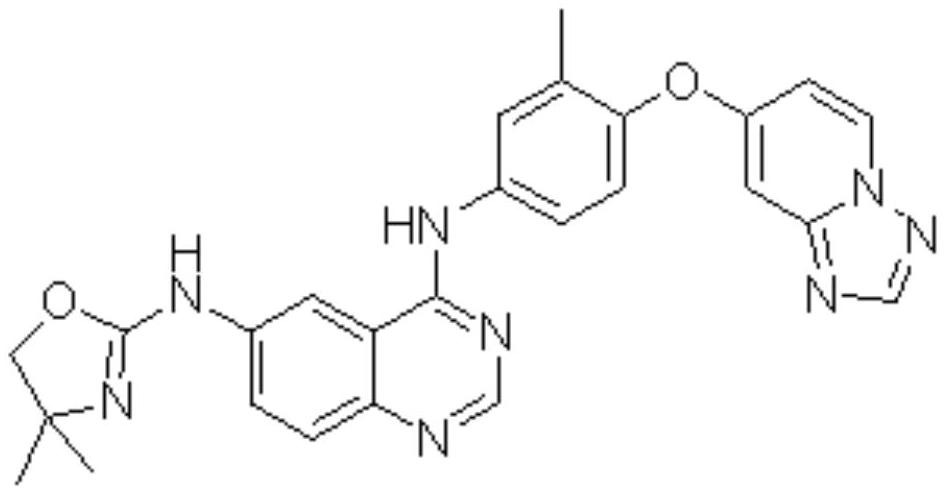
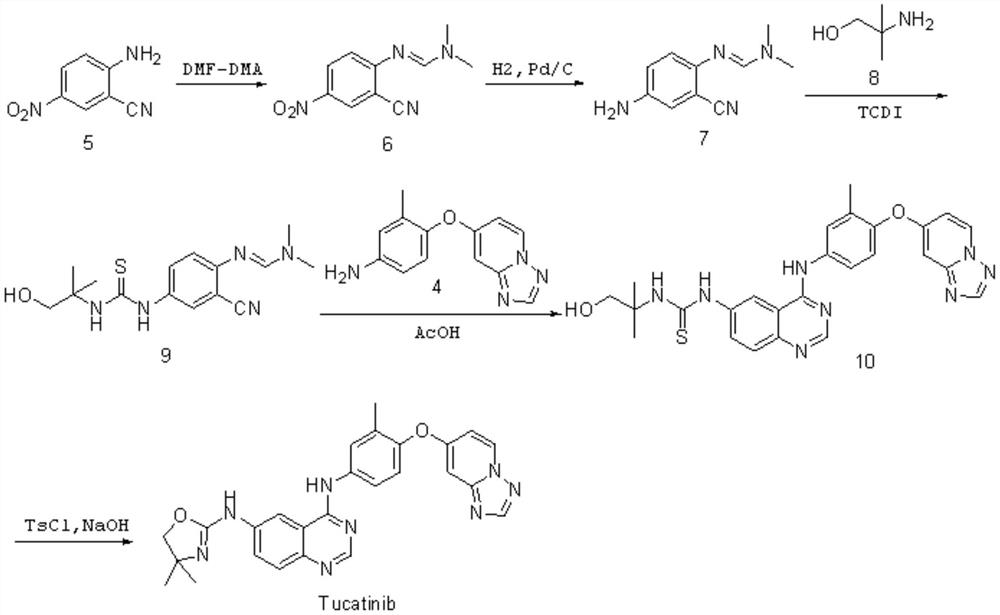
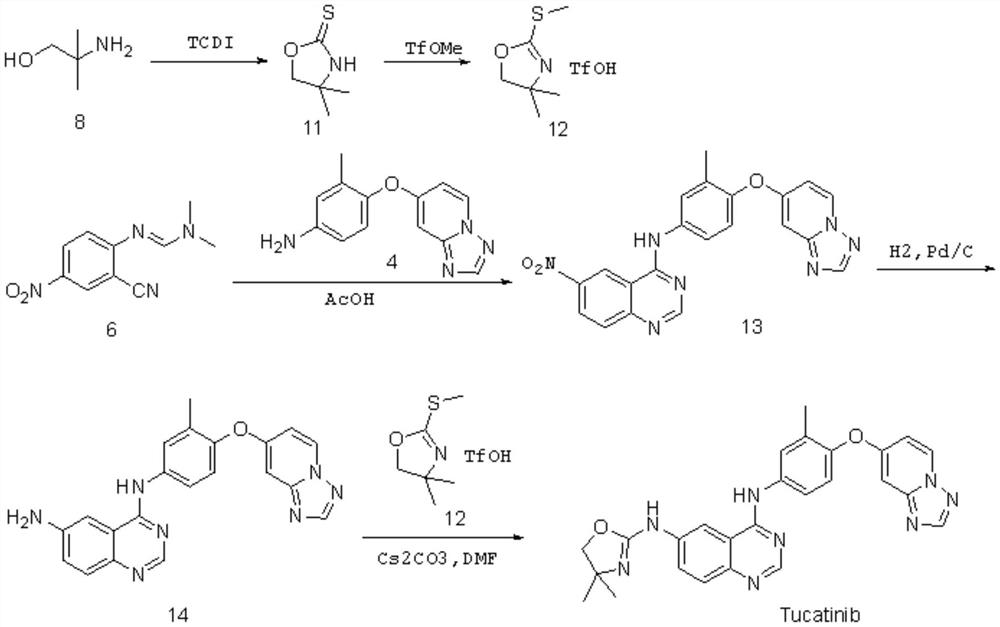
Preparation method of tucatinib
The invention relates to a preparation method of tucatinib. The method comprises the following steps: by taking 4-hydroxy-6-chloroquinazoline as a raw material, firstly carrying out copper-catalyzed C-N cross-coupling reaction on the 4-hydroxy-6-chloroquinazoline and 2-amino-4, 4-dimethyl-4, 5-dihydrooxazole, then activating the 4-hydroxy of quinazolinone by adopting a BOP, and enabling product and 4-([1, 2, 4] triazolo [1, 5-a] pyridine-7-yloxy)-3-methylaniline to be subjected to a nucleophilic substitution reaction to obtain tucatinib. With the method, using of high-corrosivity and high-toxicity reagents is avoided, and the method has low requirements on equipment, the operation difficulty and environmental protection requirements are reduced, and the method has the advantages of high product yield and high product purity; the process is simple and environment-friendly and suitable for large-scale production.
Owner:SHANDONG HUIHAI PHARMA & CHEM
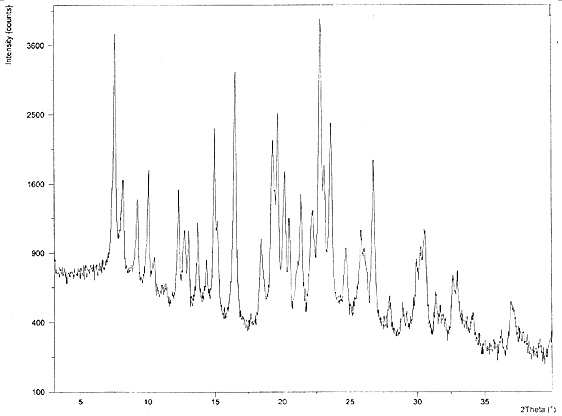
Chloroquine phosphate enantiomer crystal form and preparation method thereof
PendingCN111732539AEase of industrial productionQuality improvementAmino preparation from aminesOrganic compound preparationEnantiomerPhosphoric acid
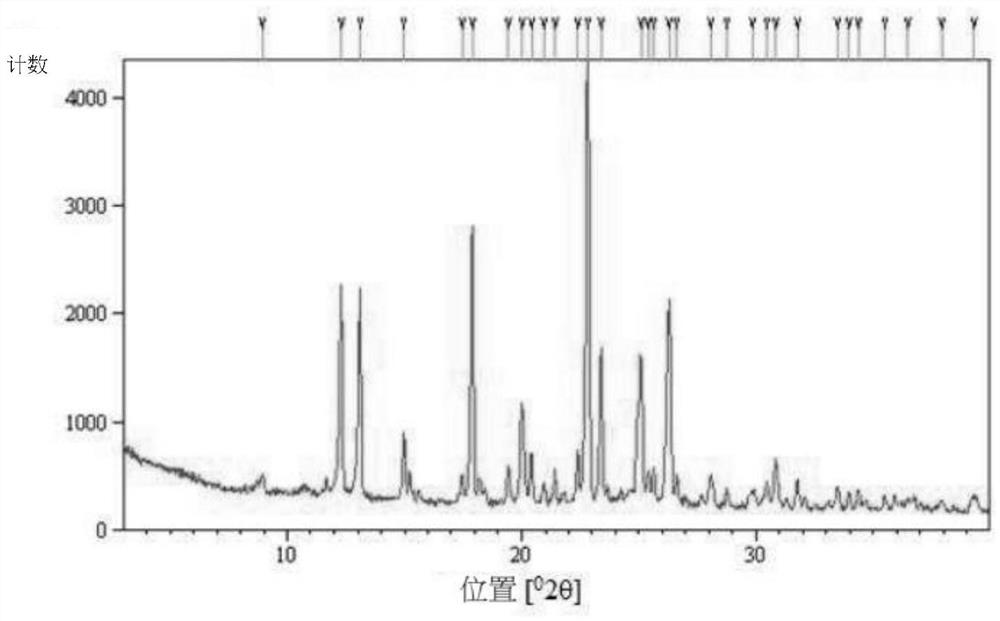
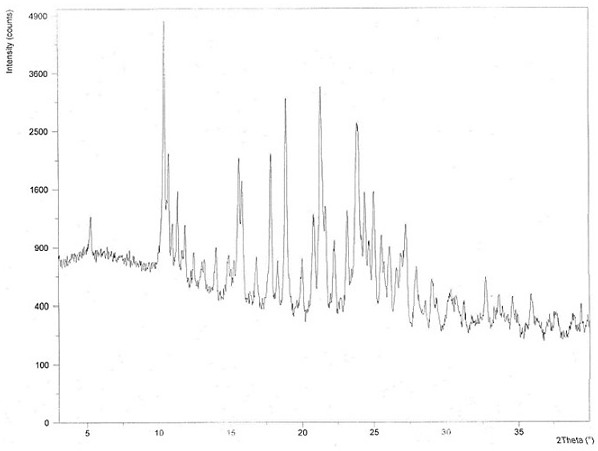
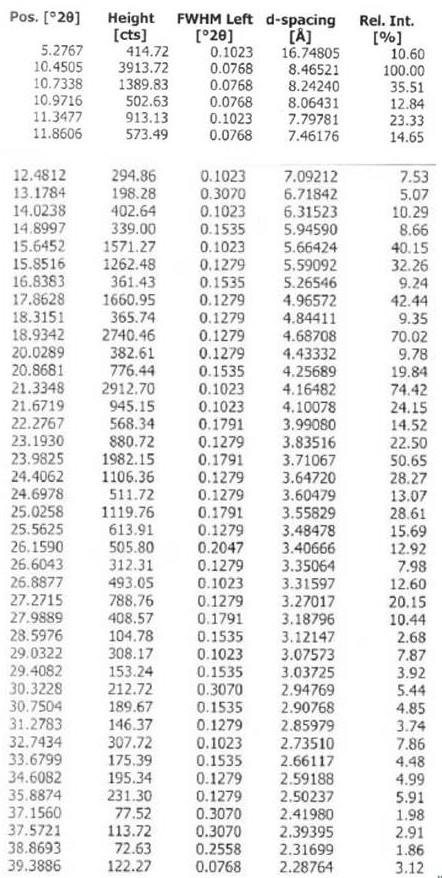
The invention discloses a chloroquine phosphate enantiomer crystal form and a preparation method thereof. The invention provides (R) / (S)-chloroquine phosphate and a preparation method thereof. According to the (S)-chloroquine phosphate provided by the invention, an X-ray powder diffraction pattern expressed by a 2theta angle has characteristic peaks at 10.4 degrees, 10.7 degrees, 15.6 degrees, 15.8 degrees, 17.8 degrees, 18.9 degrees, 21.3 degrees and 23.9 degrees + / -0.2 degrees. According to the (R)-chloroquine phosphate provided by the invention, an X-ray powder diffraction pattern expressedby a 2theta angle has characteristic peaks at 10.3 degrees, 15.5 degrees, 15.7 degrees, 17.8 degrees and 18.8 degrees + / -0.2 degrees.
Owner:珠海润都制药股份有限公司
Refining method of hydroxychloroquine crude product
The present invention provides a refining method of a hydroxychloroquine crude product. The refining method comprises two refining processes. In the first refining process, by controlling the use amount of a derivatization reagent and the derivatization reaction temperature, an impurity III is converted into a new impurity easy to remove, the new impurity is removed through a recrystallization mode, the active ingredient hydroxychloroquine hardly participates in the derivatization reaction, the primary refining of the crude hydroxychloroquine product is realized, almost no impurity III exists in the primary refined product, the loss of hydroxychloroquine is low, the yield reaches 90% or above, and the purity reaches 99.5% or above. In the second refining process, a simple recrystallization mode is adopted, the yield of the hydroxychloroquine is high, the yield reaches about 95%, and the purity reaches 99.9%.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
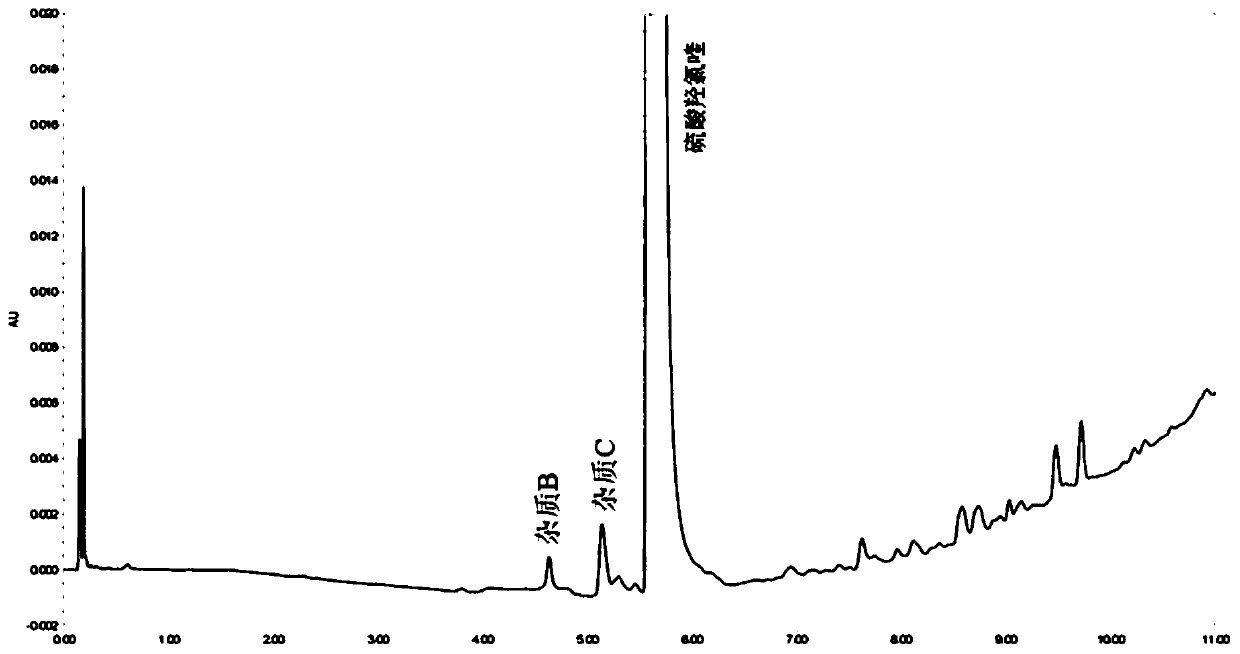
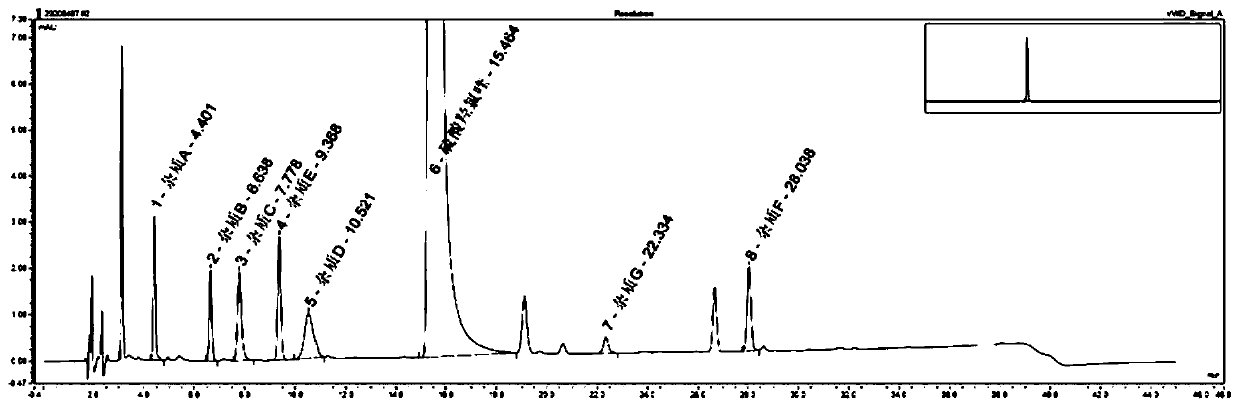
Method for detecting hydroxychloroquine sulfate related substances and application thereof
ActiveCN111551645AEasy to separateHigh sensitivityComponent separationHydroxychloroquine SulfateSolvent
The invention discloses a method for detecting hydroxychloroquine sulfate related substances. The method comprises the following step: carrying out separation and detection by adopting reverse liquidchromatography, wherein chromatographic conditions are as follows: a mobile phase is a mixed solvent consisting of a water-soluble organic solvent and a buffer solution; wherein the buffer solution isan ammonium acetate solution or an ammonium formate solution; wherein the pH value of the buffer solution is 9.0 to 10.5. The detection method disclosed by the invention can be used for simultaneously detecting seven impurities recorded in quality standards of hydroxychloroquine sulfate raw materials in European Pharmacopoeia 9.8 versions in hydroxychloroquine sulfate raw materials and preparations, and has the advantages of good separation effect, high sensitivity, no blank interference, low detection cost, high analysis speed and the like.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA
Pharmaceutical composition containing hdac6 inhibitor as active ingredient for prevention or treatment of itching
InactiveUS20210077487A1Effectively inhibiting and treating itchingOrganic active ingredientsDermatological disorderHDAC6Pharmaceutical drug
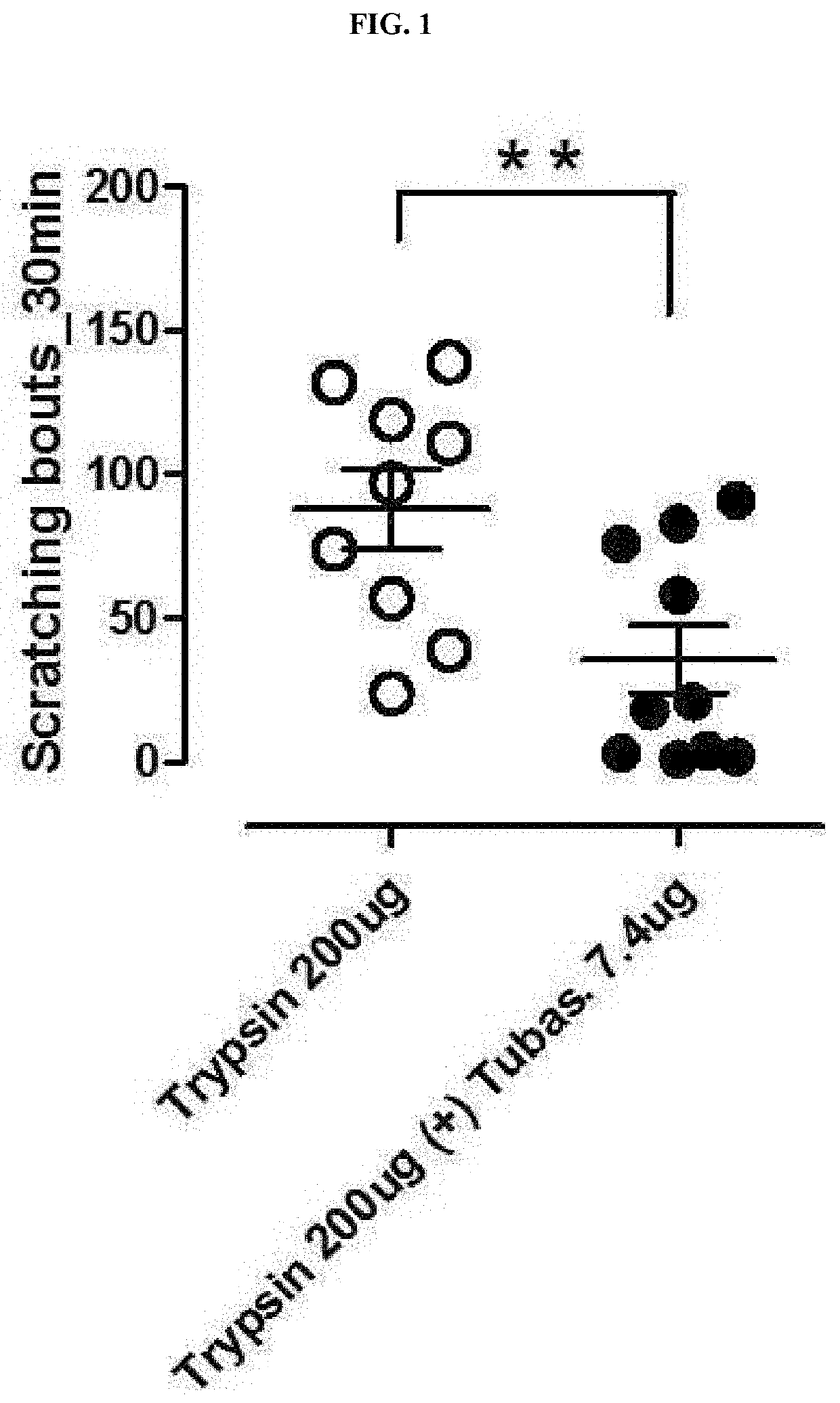
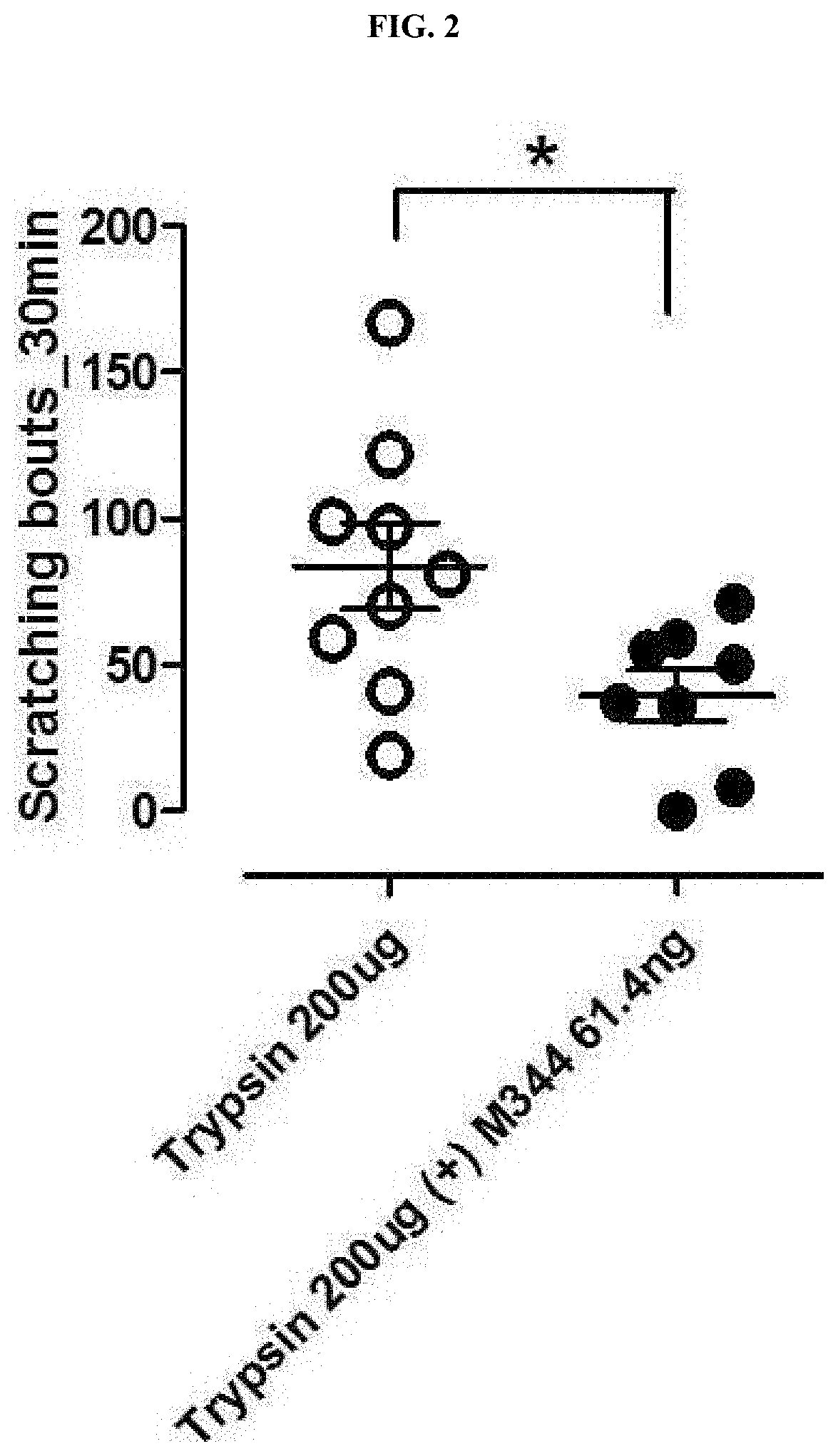
The present invention relates to a pharmaceutical composition containing an HDAC6 inhibitor as an active ingredient for prevention or treatment of itching. The present invention provides a novel pharmaceutical composition containing, as an active ingredient, an inhibitor for inhibiting an HDAC6 enzyme, and thus can effectively inhibit and treat itching caused by trypsin, tryptase, histamine, antimycin A, chloroquine, or the like.
Owner:BNH RES CO LTD
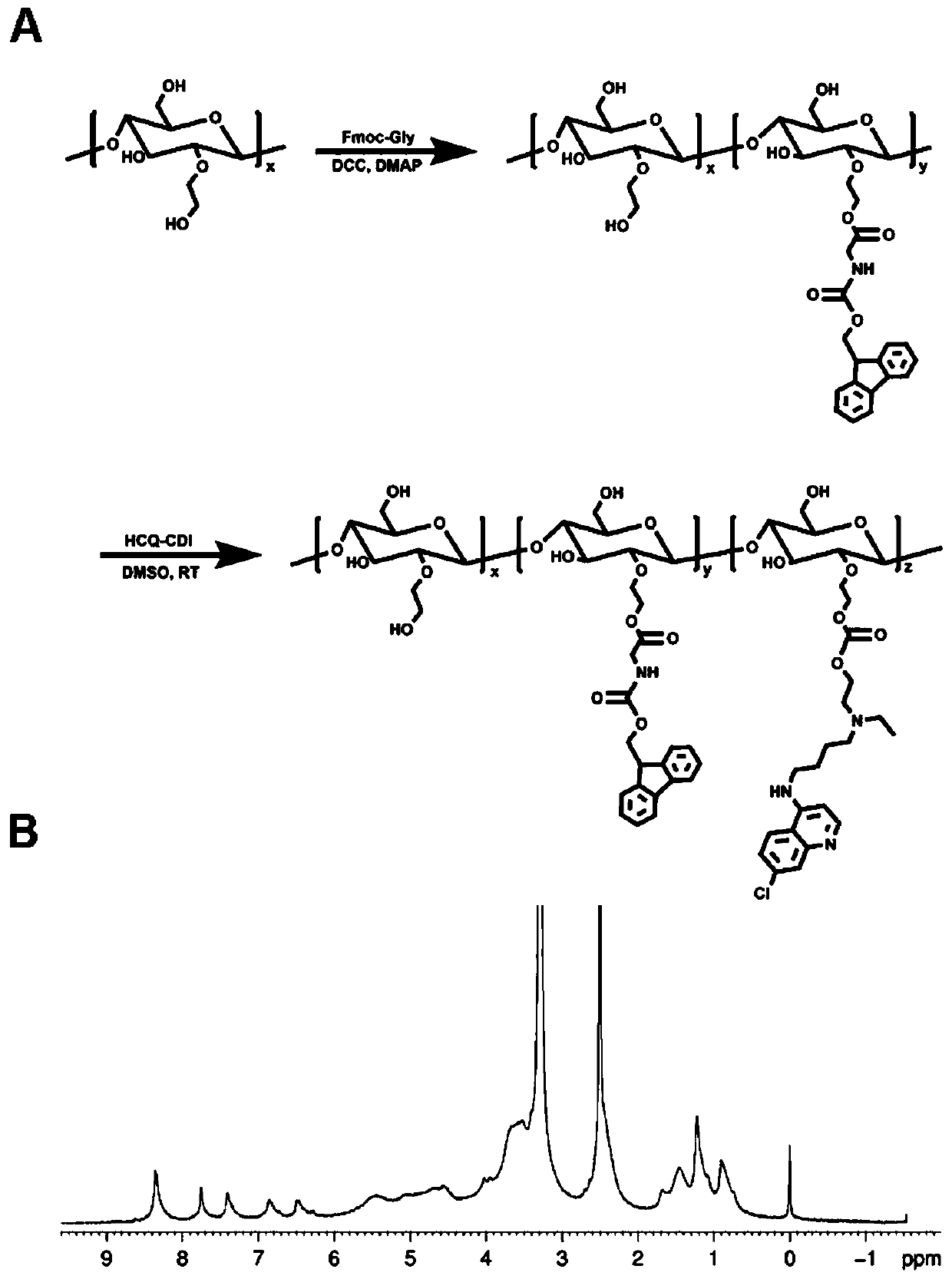
Polymerized chloroquine fluorenylmethyl nanogel delivery system and preparation method thereof
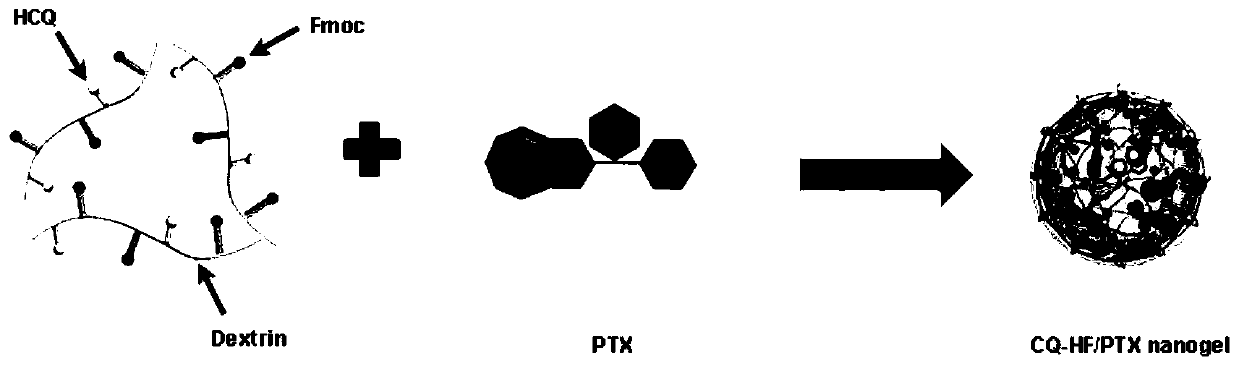
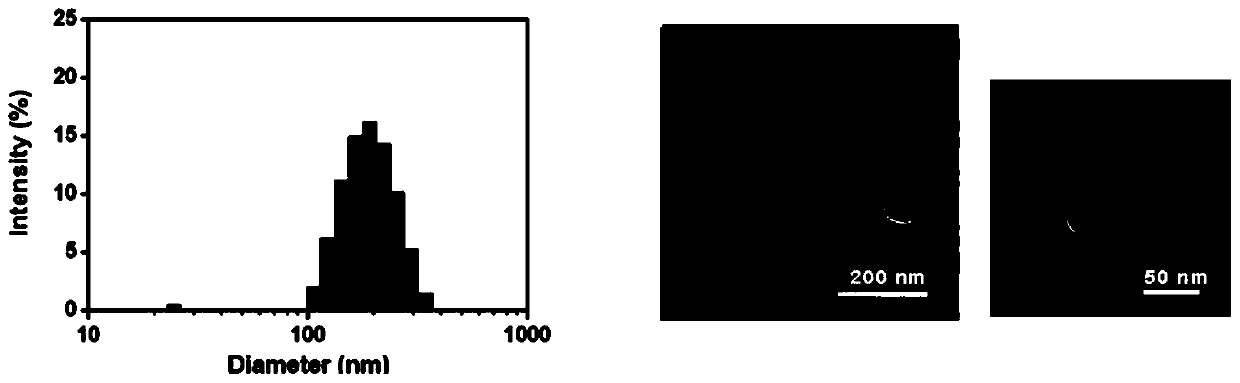
ActiveCN111249473AInhibit transferPrevent proliferationOrganic active ingredientsPowder deliveryHydroxychloroquineEngineering
The invention discloses a polymerized chloroquine fluorenylmethyl nanogel delivery system. The delivery system comprises nanogel particles of polymerized chloroquine fluorenylmethyl and an anti-tumordrug wrapped in the particles. The nanogel particles are formed by crosslinking a polysaccharide main chain modified with hydroxychloroquine and fmoc-D-methionine, the molecular weight of the polysaccharide main chain is 5-100 kD, the molar substitution degree of the hydroxychloroquine on the polysaccharide main chain is 5%-50%, and the molar substitution degree of the fmoc-D-methionine on the polysaccharide main chain is 1%-30%. The polymerized chloroquine fluorenylmethyl nanogel delivery system can inhibit the autophagy level in cells and block a signal channel of tumor cell metastasis, meanwhile, entrap an antitumor drug paclitaxel through the pi-pi conjugation effect, and inhibit metastasis and proliferation of malignant tumors at the same time.
Owner:CHINA PHARM UNIV
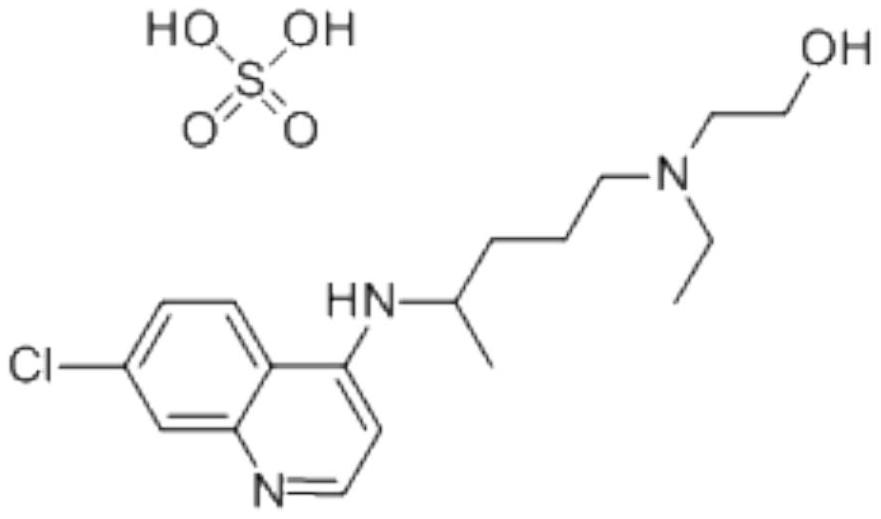
Hydroxychloroquine sulfate and preparation method thereof
PendingCN113185459AGood pollution effectSpeed up the reaction processOrganic chemistryHydroxychloroquinePtru catalyst
The invention discloses hydroxychloroquine sulfate and a preparation method thereof, and relates to the technical field of medicinal chemistry. The preparation method comprises the steps of mixing 4, 7-dichloroquinoline with a hydroxychloroquine side chain, carrying out heating condensation in the presence of an organic base catalyst, adding water and liquid, and carrying out cooling crystallization to obtain hydroxychloroquine; and dissolving hydroxychloroquine in an ethyl acetate and ethanol aqueous solution, heating, dissolving and clarifying, dropwise adding concentrated sulfuric acid, cooling, crystallizing, filtering and drying to obtain hydroxychloroquine sulfate. The method has the beneficial effects that a solvent-free reaction is used, and a catalyst is added, so that high pollution is avoided, the reaction process is accelerated, and the operation is simple; and meanwhile, the HPLC purity of the obtained hydroxychloroquine refined product is not less than 96.50%, the maximum single impurity content is less than 0.10%, the yield can reach 85%, the HPLC purity of hydroxychloroquine sulfate is not less than 98.00%, the maximum single impurity content is less than 0.10%, and the yield can reach 90%.
Owner:JIANGXI GUOYAO PHARMA LLC +1
Hydroxychloroquine sulfate pharmaceutical preparation
PendingCN113244180ASolve the problem of easy stickingSolve the problem of poor compressibility caused by high friabilityOrganic active ingredientsAntipyreticDrugs preparationsHydroxychloroquine Sulfate
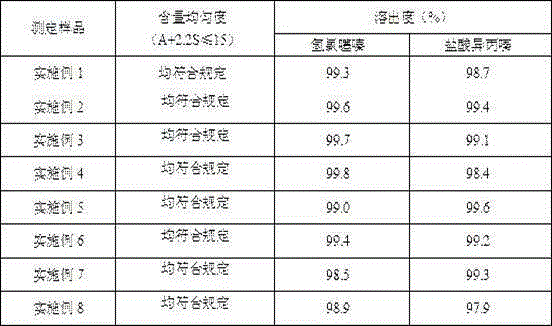
The invention provides a hydroxychloroquine sulfate pharmaceutical preparation. The hydroxychloroquine sulfate pharmaceutical preparation comprises the following components in percentage by weight: 50-75% of hydroxychloroquine sulfate, 5-15% of mannitol, 15-35% of pregelatinized starch, 0.5-2.0% of a water-soluble adhesive and 0.5-2.0% of a lubricant. According to the hydroxychloroquine pharmaceutical preparation disclosed by the invention, the mannitol and the pregelatinized starch are used as filling agents, and are matched with the adhesive and the lubricant, so that the problem that the hydroxychloroquine pharmaceutical preparation is extremely likely to stick and the problem of poor compressibility caused by high friability of tablets can be solved, the hydroxychloroquine pharmaceutical preparation has good dissolution property, and the dissolution effect is consistent with that of a reference reagent.
Owner:BEIJING WINSUNNY PHARMA CO LTD
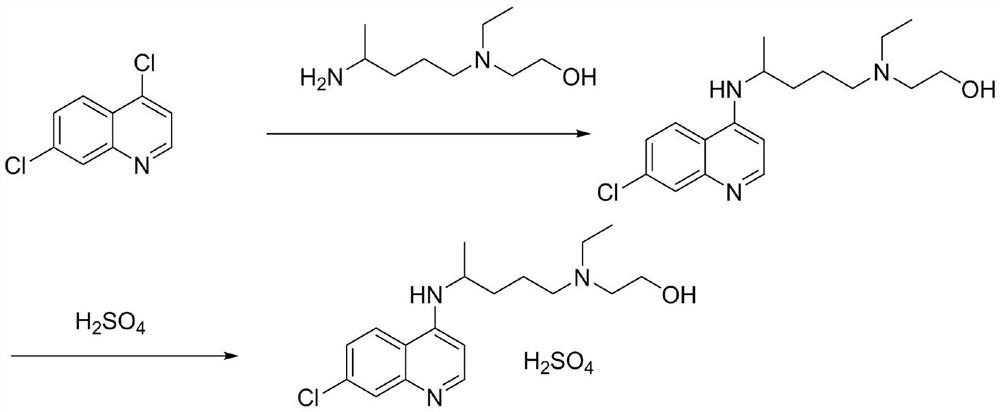
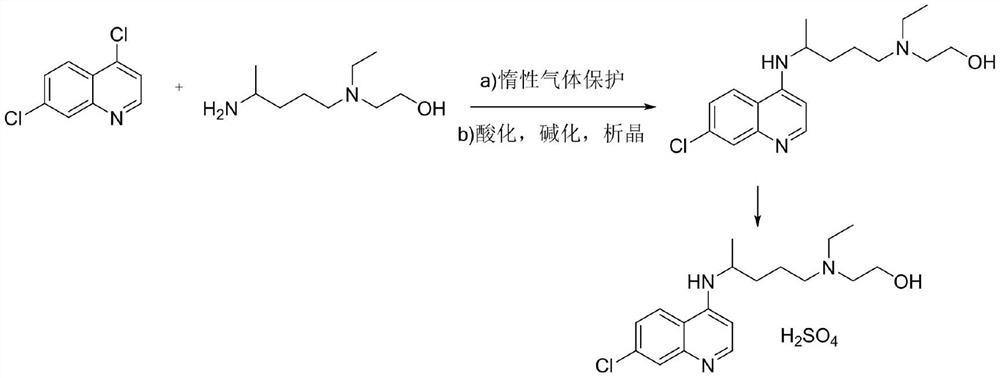
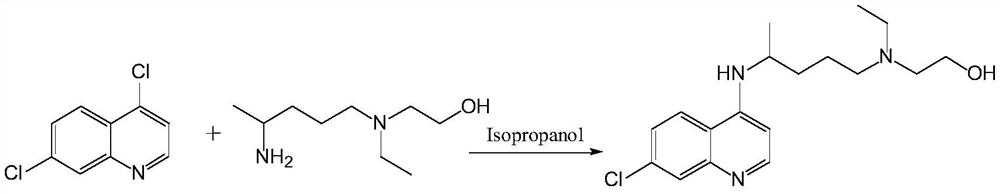
Preparation method of chloroquine phosphate
The invention discloses a preparation method of chloroquine phosphate, namely 7-chloro-4-(4-diethylamino-1-methylbutylamino)quinoline diphosphate. The preparation method comprises the following steps: (1) carrying out a condensation reaction on 4,7-dichloroquinoline and 2-amino-5-diethylaminopentane, and carrying out alkalization extraction, concentration and crystallization to obtain chloroquine; and (2) salifying the chloroquine obtained in the step (1) and phosphoric acid to obtain chloroquine phosphate. The method avoids the use of phenol, is simple in reaction, and is beneficial to industrial production; and the chloroquine is crystallized to improve the purity and then salified with the phosphoric acid, so product appearance is good, the purity of the produced chloroquine phosphate is greater than or equal to 99.5%, and a single impurity is less than 0.1% (according to an HPLC area normalization method).
Owner:CHONGQING KANGLE PHARMA
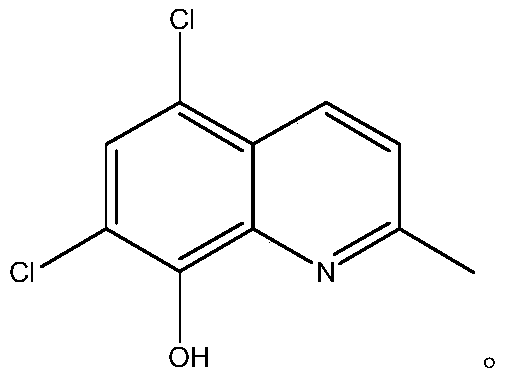
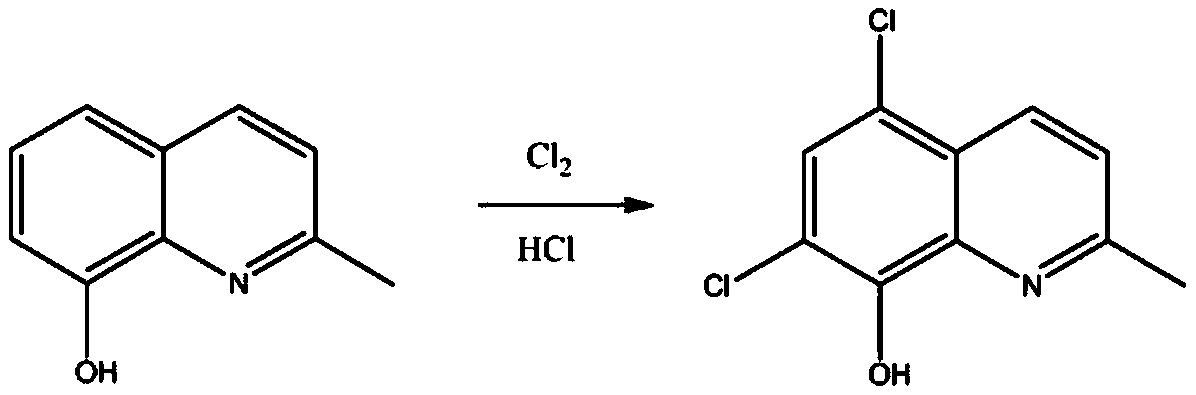
Preparation method of chloroquinaldol
ActiveCN111116467AHigh purityImprove stabilityOrganic chemistryBiochemical engineeringProcess engineering
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of chloroquinaldol. The preparation method comprises the following steps: adding water intochloroquinaldol hydrochloride for dissolving, adding a buffer solution under the conditions of light shielding and nitrogen protection, regulating the pH value by using alkali, filtering and refiningthe solution to obtain chloroquinaldol. The method is easy and convenient to operate and suitable for industrial production, the purity of the prepared chloroquinaldol is high and can reach 99.99% orabove, and the stability of the product is high.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
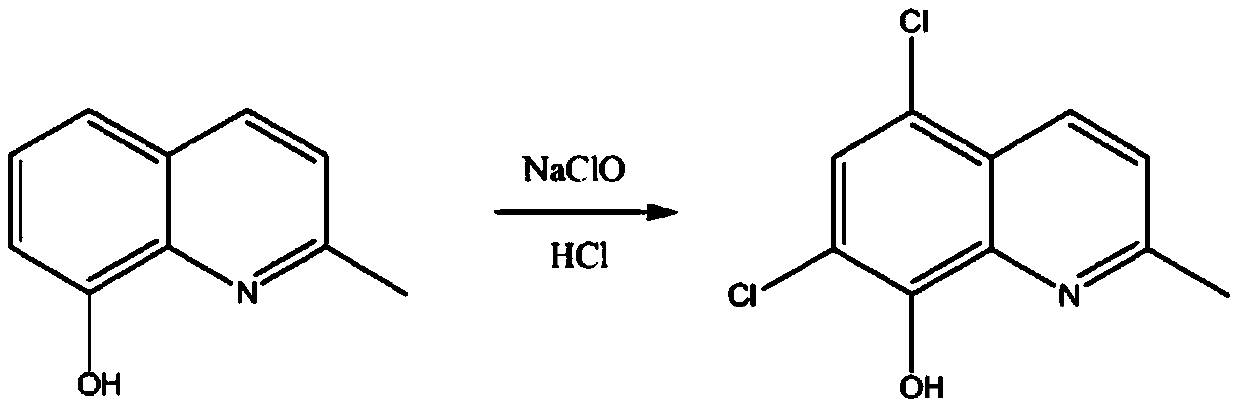
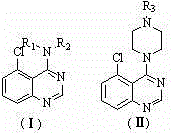
4-N-substituted-5-chloroquinazoline compound and preparation method and application thereof
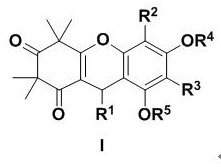
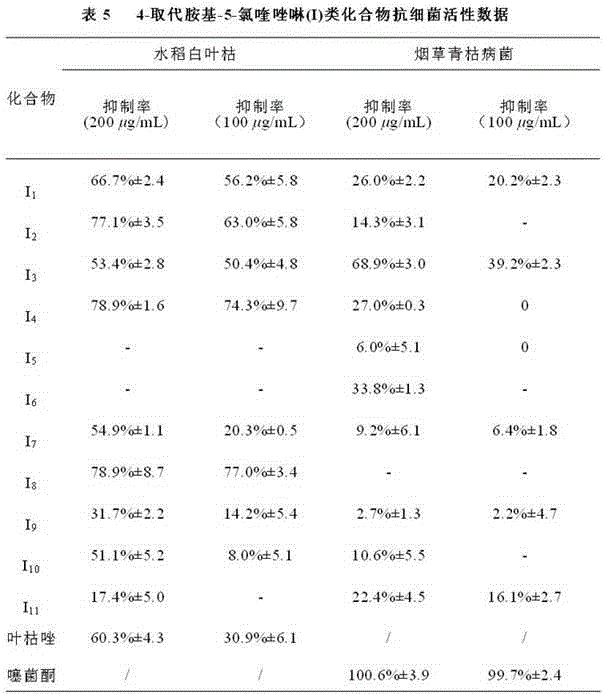
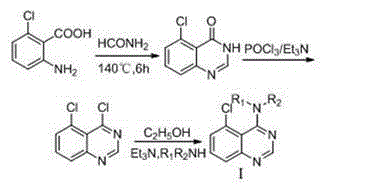
The invention discloses a preparation method and biological activity of a compound with a function of resisting plant germs, 4-N-substituted-5-chloroquinazoline compound which is a compound represented by the general formulas (I) and (II) and a preparation method thereof. According to the invention, the 4-N-substituted-5-chloroquinazoline derivative is synthesized via three or five steps by adopting 2-amino-6-chlorobenzoic acid, formamide, phosphoryl chloride, concentrated hydrochloric acid, N-Boc piperazine and substituted benzyl chloride or benzyl bromine as raw materials and adopting sodium hydride, triethylamine and potassium carbonate as catalysts. The compounds I2, I4, I8 and II5 disclosed by the invention have relatively good inhibiting effects on plant fungi, and the compounds II1, II7, II10, II11, II16 and II17 show relatively high bacteriostatic activity on plant bacteria.
Owner:GUIZHOU UNIV
Preparation method of hydroxychloroquine
ActiveCN111635358AMeet high purity quality requirementsAvoid generatingOrganic chemistryBulk chemical productionBenzoic acidHydroxychloroquine
The invention belongs to the technical field of medicine and chemical engineering, and particularly relates to a hydroxychloroquine preparation method. The method comprises: carrying out a condensation reaction on a quinoline intermediate 7-chloro-4-hydroxyquinoline sulfonate and a hydroxychloroquine side chain in a eutectic solvent to obtain a target product, wherein the preparation method of thequinoline intermediate 7-chloro-4-hydroxyquinoline sulfonate comprises the following steps: (1) by taking 4-chloro-2-nitrobenzoic acid as a raw material, carrying out a chlorination reaction to prepare acyl chloride, condensing the acyl chloride with Meldrum's acid, and hydrolyzing to obtain 4-chloro-2-nitroacetophenone; and (2) carrying out condensation reaction, nitro reduction cyclization andhydroxyl protection reaction on the 4-chloro-2-nitroacetophenone and N,N-dimethylformamide methylal to obtain the quinoline intermediate 7-chloro-4-hydroxyquinoline sulfonate. The method has the advantages of easily available raw materials, mild reaction conditions, difficulty in side reaction, avoidance of high-temperature production conditions, reduction of risks, good intermediate stability, high yield and good purity of the obtained hydroxychloroquine, and facilitation of large-scale production.
Owner:北京成宇化工有限公司
Hydroxychloroquine sulfate as well as crystal form and preparation method of enantiomer of hydroxychloroquine sulfate
PendingCN111793026AGood chemical stabilityReduce riskOrganic compound preparationOrganic chemistry methodsNitrogen oxidesEnantiomer
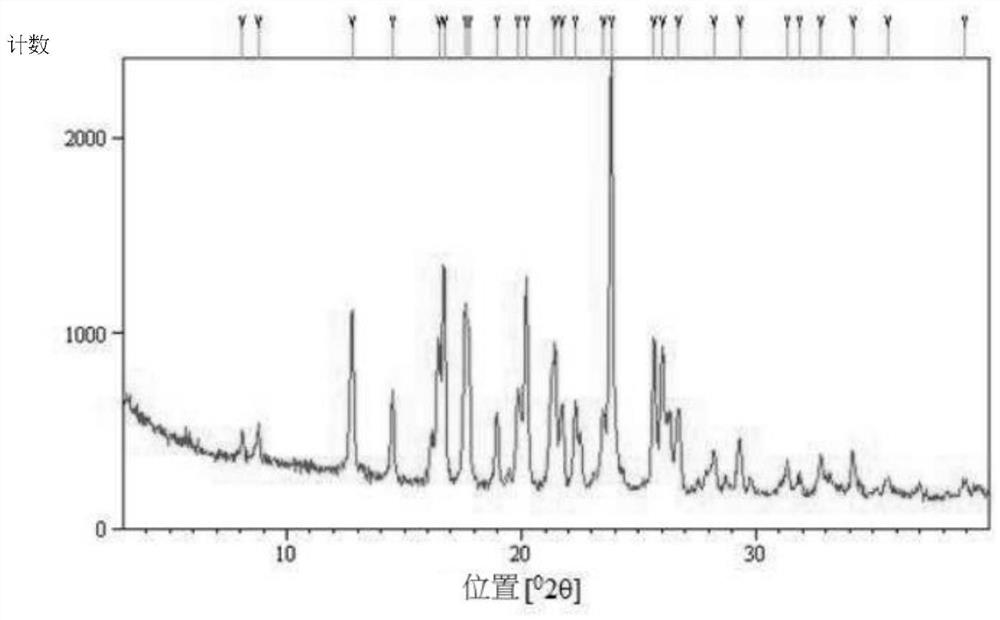
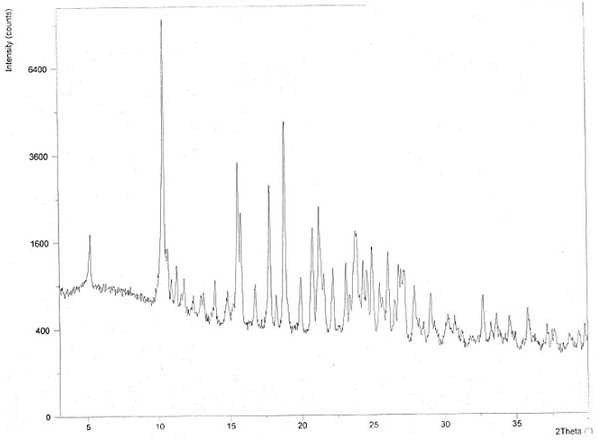
The invention relates to hydroxychloroquine sulfate as well as a crystal form and a preparation method of an enantiomer of hydroxychloroquine sulfate. The invention provides a crystal form A hydroxychloroquine sulfate. An X-ray powder diffraction pattern of the crystal form A hydroxychloroquine sulfate has characteristic peaks at 10.8 degrees, 13.0 degrees, 13.3 degrees, 16.9 degrees, 17.2 degrees, 17.5 degrees, 19.9 degrees, 21.3 degrees, 23.5 degrees, 24.0 degrees and 26.7 degrees + / -0.2 degrees, and does not contain hydroxychloroquine nitrogen oxide; the invention provides a hydroxychloroquine crystal, the X-ray powder diffraction pattern has characteristic peaks at 7.5 degrees, 14.9 degrees, 16.5 degrees, 19.2 degrees, 19.6 degrees, 22.8 degrees, 23.6 degrees and 26.7 degrees+ / -0.2 degree; the invention provides an S-hydroxychloroquine sulfate monohydrate, the X-ray powder diffraction pattern has characteristic peaks at 12.2 degrees, 13.0 degrees, 14.9 degrees, 17.8 degrees, 22.7 degrees, 23.3 degrees, 25.0 degrees and 26.2 degrees + / -0.2 degrees; the invention also provides R-hydroxychloroquine sulfate, the X-ray powder diffraction pattern has characteristic peaks at 12.2 degrees, 13.0 degrees, 14.9 degrees, 17.8 degrees, 22.7 degrees, 23.3 degrees, 25.0 degrees and 26.2 degrees + / -0.2 degrees.
Owner:珠海润都制药股份有限公司
Chloroquine-coated denatured albumin nanoparticles for selectively resisting inflammatory cells as well as preparation method and application of chloroquine-coated denatured albumin nanoparticles
PendingCN114146065AImprove stabilityGood compatibilityOrganic active ingredientsAntipyreticNanoparti clesDextran
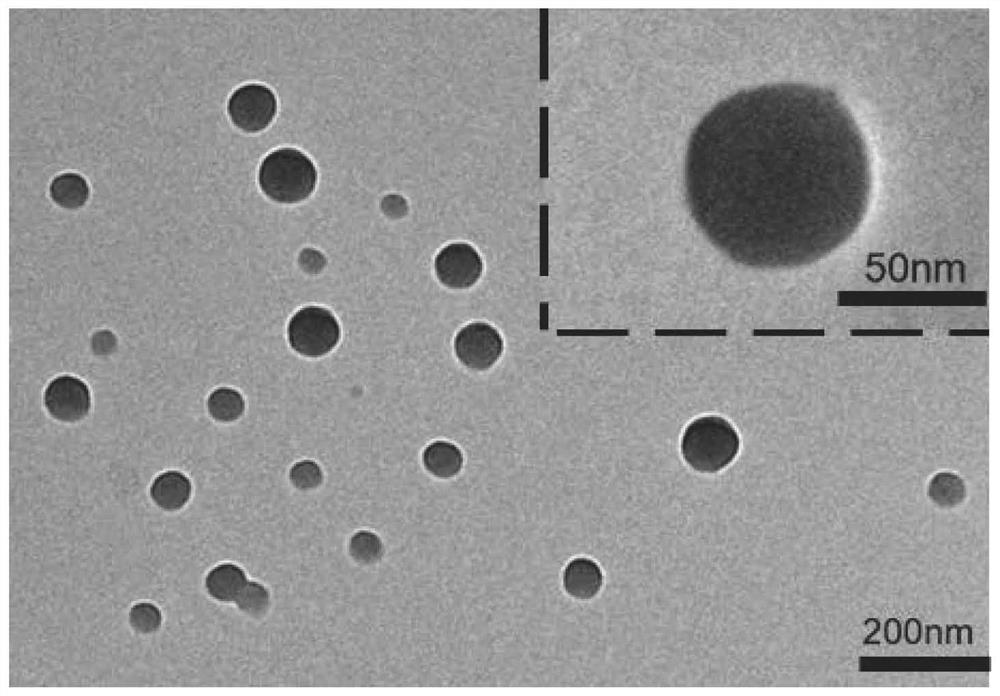
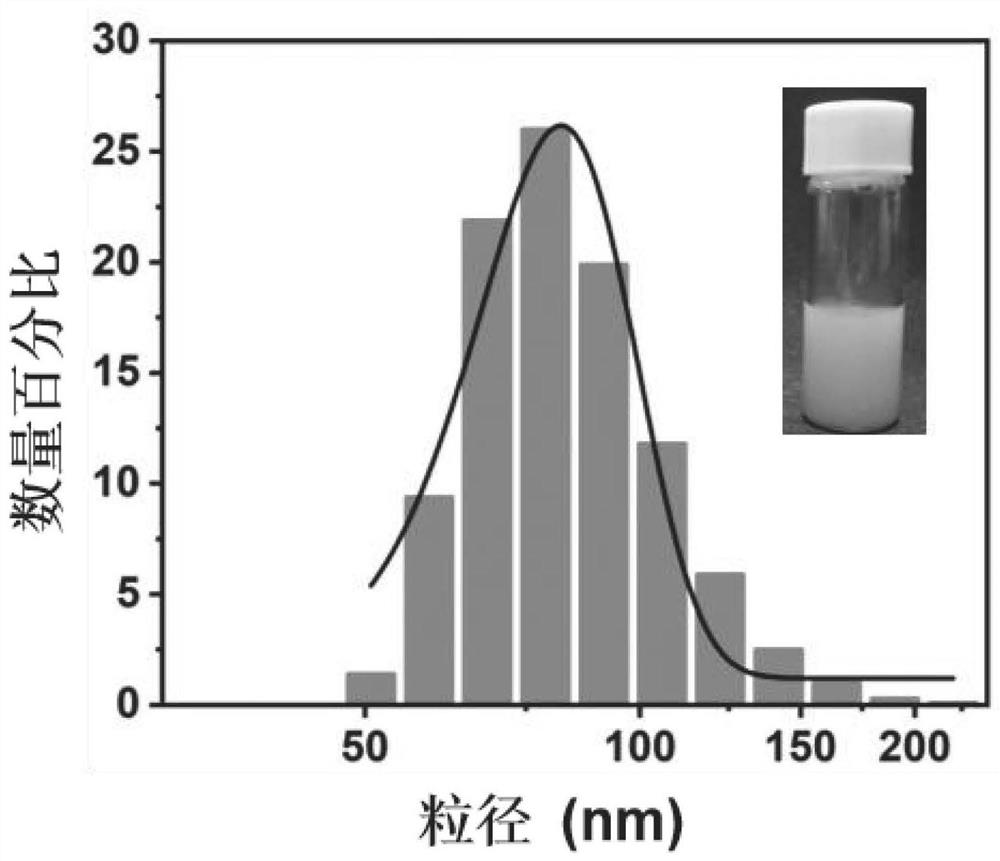
The invention discloses a chloroquine-coated denatured albumin nanoparticle for selectively resisting inflammatory cells as well as a preparation method and application thereof, and the denatured albumin nanoparticle is used as a carrier and is coated with a drug chloroquine. The nanoparticles have good stability and biocompatibility, can be efficiently internalized by macrophages, and can effectively aim at inflammatory cells at colon parts, so that inflammation is relieved, and the nanoparticles are used for treating colitis diseases caused by dextran sodium sulfate.
Owner:HEFEI UNIV OF TECH
Chloroquine phosphate inhalation aerosol and preparation method thereof
InactiveCN111110634AImprove stabilityAccurate doseOrganic active ingredientsDispersion deliveryInhalationPhosphoric acid
The invention belongs to the technical field of medicine, and discloses a chloroquine phosphate inhalation aerosol and a preparation method thereof. The chloroquine phosphate inhalation aerosol is composed of active ingredient chloroquine phosphate and a certain proportion of a propellant, a flavoring agent, a pH regulator and water for injection. The aerosol inhaler is administered through mouthsand directly acts on lungs to realize targeted drug delivery. The inhalation preparation provided by the invention can target lesion, has an accurate dosage and a fast effect, can quickly improve theinfection conditions of the lungs, facilitates improving the adaptability of infected people, and avoids absorption through gastrointestinal tracts to reduce gastrointestinal side effects.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com