Medicine composition for treating pituitary adenoma, application of medicine composition, medicine box and packaging piece
A technology of pituitary adenoma and composition, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
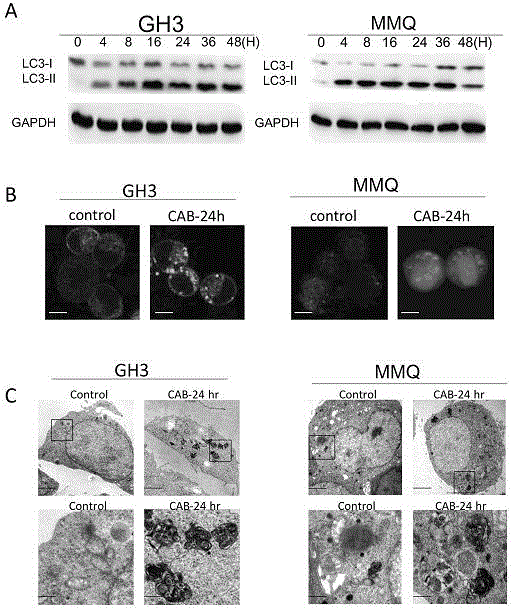
[0032] Example 1, Cabergoline-induced autophagy in rat MMQ and GH3 cells
[0033] Experimental Materials:
[0034] Rat pituitary tumor cell line MMQ (ATCC) at 37 °C, 5 % CO 2 Under normal conditions, the culture medium was DMEM / F12 (Gibco) containing 2.5% fetal bovine serum (Gibco) plus 12.5% horse serum; cabergoline was purchased from Tocris (UK), and the mother solution 100mM was prepared by DMSO; GFP-LC3 Plasmids were from Addgene. Chloroquine (CQ) was purchased from sigma (USA), and the mother solution was prepared with 100 mM ultrapure water; MTS and ATP detection cell viability reagents were purchased from Promega (USA); RIPA lysate and PMSF were purchased from Beyond Biotechnology Research Institute (Jiangsu) ; Protease Inhibitor Cocktail was purchased from Merck (Germany).
[0035] experimental method:
[0036] 1) Cell culture and administration
[0037] Cells were suspended in regular culture medium and the cell density was adjusted to 1×10 6 Cells / ...
Embodiment 2
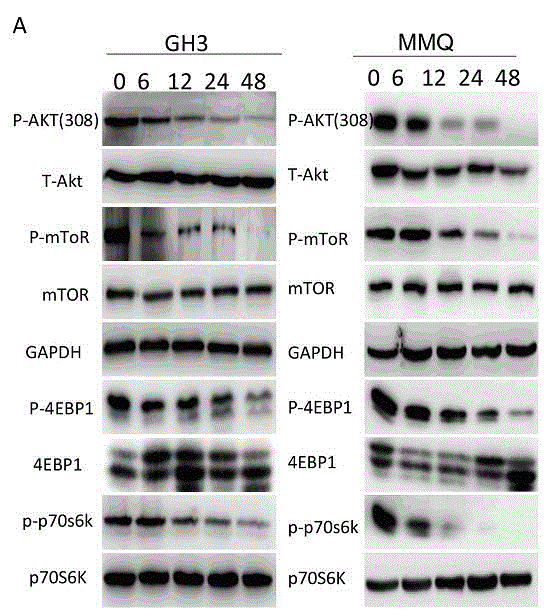
[0059] Example 2, Cabergoline inhibits mTOR signaling pathway experiment
[0060] Experimental material: with embodiment 1.
[0061] Western Blot detection of expression of Polyclonal anti-phospho-AKT, anti-AKT, anti-phospho-p70s6k, anti-p70s6k, anti-phospho-4EBP1, anti-4EBP1, anti-phospho-mTOR, and anti-mTOR;
[0062] The experimental method is the same as the protein detection method in the implementation case 1, figure 2 Selected representative experimental results are shown, and the results show that cabergoline has an inhibitory effect on the mTOR pathway on MMQ cells.
Embodiment 3
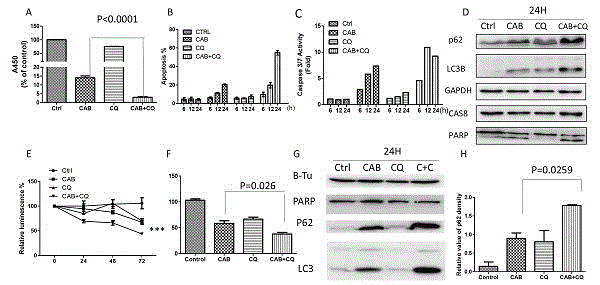
[0063] Example 3, In Vitro Test of Chloroquine Combined with Cabergoline
[0064] 1. MMQ cell MTS assay to detect the efficacy of CAB+CQ (such as image 3 As shown in A)
[0065] 1) Suspend the cells in regular culture medium and adjust the cell density to 1×10 4 The cells / well were inoculated in a 96-well culture plate, and the drug-containing medium was added to divide into 4 groups, namely DMSO, CAB, CQ and CAB+CQ groups, with 5 secondary wells in each group, so that the final concentration of cabergoline was 50 μM. The final concentration of CQ was 20uM, and the blank control group contained 0.3% DMSO;
[0066] 2) Culture the administered cells for 24 hours at 37 °C, 5% CO2;
[0067] 3) Add 20 μl MTS solution to each well, and continue culturing in the incubator for 4 hours;
[0068] 4) Measure the absorbance value with a detection wavelength of 490nm, calculate the growth inhibition rate of the cells, N=5, and run the experiment in parallel three times;
[0069] 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com