Preparation method of chloroquine phosphate
A technology of chloroquine phosphate and phosphoric acid, applied in the fields of medicine and chemical industry, can solve the problems of uneven mixing, toxic and corrosive phenol, inconvenient use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
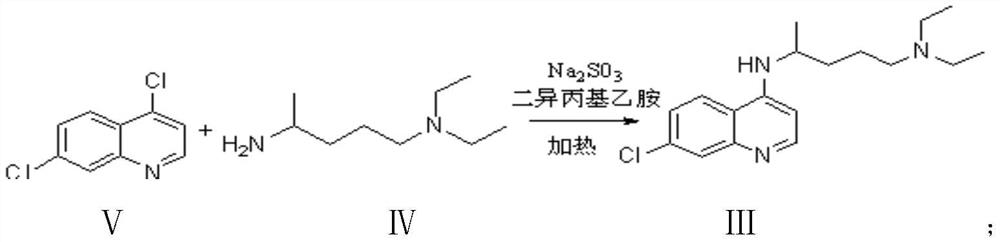
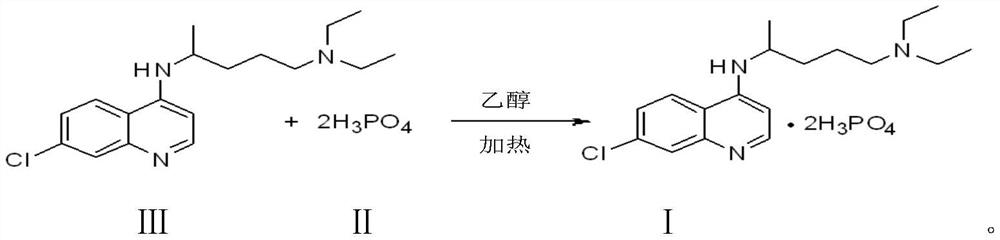
[0030] The preparation of embodiment 1 chloroquine phosphate formula I
[0031] Condensation reaction:
[0032] Add 103.0 g of 4,7-dichloroquinoline, 3.3 g of sodium sulfite, 24.2 g of N,N-diisopropylethylamine, and 600.0 g of isopropanol into the reaction flask, start stirring, and heat up to 4,7-dichloroquinoline to dissolve ; Add 95.5 g of 2-amino-5-diethylaminopentane dropwise; slowly heat up, and the isopropanol is evaporated. When the internal temperature rises to 133.0°C; start the timing reaction. The internal temperature of the reaction was controlled at 133.0°C to 138.0°C, and the heat preservation reaction was carried out for a total of 10 hours.
[0033] Alkaline Extraction:
[0034] Add 100.0g of drinking water to the alkalization bottle, start stirring; slowly pour the reaction solution into the alkalization bottle, add sodium hydroxide, adjust the pH of the water layer to 12; minutes, the alkalization is completed; the feed liquid is cooled to 70.0°C. 300.0 ...
Embodiment 2
[0039] The preparation of embodiment 2 chloroquine phosphate formula I
[0040] Condensation reaction:
[0041] Add 103.0 g of 4,7-dichloroquinoline, 13.1 g of sodium sulfite, 11.4 g of N,N-diisopropylethylamine, and 600.0 g of isopropanol into the reaction flask, start stirring, and heat up to 4,7-dichloroquinoline to dissolve ; Add 83.1 g of 2-amino-5-diethylaminopentane dropwise; slowly heat up, and the isopropanol is evaporated. When the internal temperature rises to 133.0°C; start the timing reaction. The inner temperature of the reaction was controlled at 133.0°C to 138.0°C, and the heat preservation reaction was carried out for a total of 12 hours.
[0042] Alkaline Extraction:
[0043] Add 100.0g of drinking water to the alkalization bottle, start stirring; slowly pour the reaction solution into the alkalization bottle, add sodium hydroxide, adjust the pH of the water layer to 11; minutes, the alkalization is completed; the feed liquid is cooled to 70.0°C. 300.0 g ...
Embodiment 3
[0048] The preparation of embodiment 3 chloroquine phosphate formula I
[0049] Condensation reaction:
[0050] Add 103.0 g of 4,7-dichloroquinoline, 6.5 g of sodium sulfite, 16.1 g of N,N-diisopropylethylamine, and 600.0 g of isopropanol into the reaction flask, start stirring, and heat up to 4,7-dichloroquinoline to dissolve ; Add 87.5 g of 2-amino-5-diethylaminopentane dropwise; slowly raise the temperature, and when the isopropanol is evaporated, the internal temperature rises to 133.0°C; start the timing reaction. The inner temperature of the reaction was controlled at 133.0°C to 138.0°C, and the heat preservation reaction was carried out for a total of 12 hours.
[0051] Alkaline Extraction:
[0052]Add 100.0g of drinking water into the alkalization bottle, start stirring; slowly pour the reaction solution into the alkalization bottle, add sodium hydroxide, adjust the pH of the water layer to 12; minutes, the alkalization is completed; the feed liquid is cooled to 70....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com