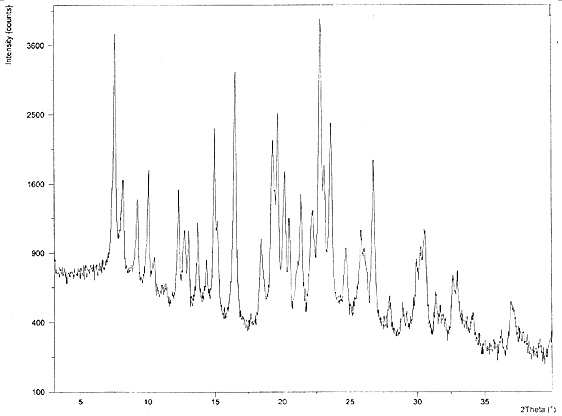
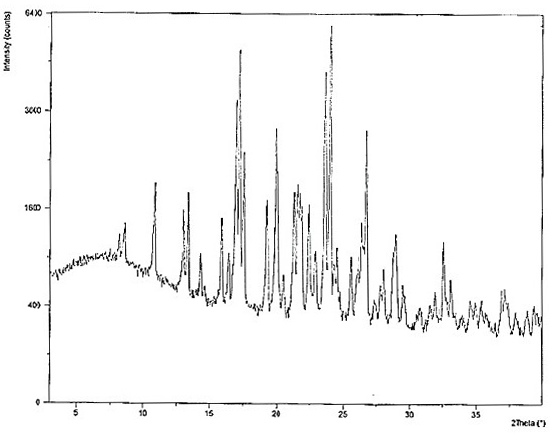
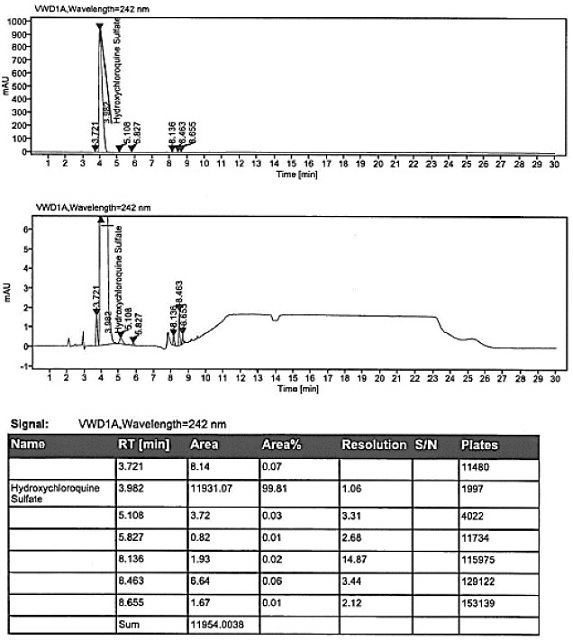
Hydroxychloroquine sulfate as well as crystal form and preparation method of enantiomer of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine is applied in the crystal field of S-hydroxychloroquine sulfate, R-hydroxychloroquine sulfate and hydroxychloroquine sulfate, and can solve the problems of low yield, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The preparation of embodiment 1 hydroxychloroquine
[0117] Weigh 100g of 4,7-dichloroquinoline, 105.6g of hydroxychloroquine side chain, and 25g of isopropanol in a 500ml three-neck flask, raise the temperature to 90°C and keep it for 30min, then raise the temperature to 95°C for 24h; cool down to 70°C, drop 5% sodium hydroxide solution (prepared from 13g of sodium hydroxide and 247g of water), add 350g of dichloromethane and stir for 5min, let stand for liquid separation, concentrate the dichloromethane layer under reduced pressure; add 450g of ethyl acetate and 50g of isocyanate to the concentrate Propanol, stirred and dissolved, crystallized at 20-30°C for 6h, suction filtered, and the filter cake was dried at 55-65°C for 6h, and 118g of hydroxychloroquine was obtained with a purity of 99.5%.
Embodiment 2
[0118] The preparation of embodiment 2 hydroxychloroquine sulfate
[0119] Add 118g of hydroxychloroquine obtained in Example 1 into a 1000ml three-neck bottle, then add absolute ethanol with a weight ratio of 4 times, stir to dissolve, add sulfuric acid solution (mass fraction concentration: 47%) dropwise at 15-25°C, and adjust pH=2 , after stirring for 3 hours, slowly cooling down to 10-15°C to crystallize for 6 hours, filtering, and placing the wet product in a vacuum drying oven at 60°C for 8 hours to obtain 122 g of hydroxychloroquine sulfate with a purity of 99.81%, and no hydroxychloroquine nitrogen oxide was detected.
Embodiment 3
[0120] The preparation of embodiment 3 hydroxychloroquine
[0121] Weigh 100g of 4,7-dichloroquinoline, 110g of hydroxychloroquine side chain, and 30g of isopropanol in a 500ml three-necked flask, raise the temperature to 90°C and keep it for 30min, then raise the temperature to 100°C for 24h; cool down to 80°C, drop 5 % sodium hydroxide solution (prepared from 13g of sodium hydroxide and 247g of water), add 350g of dichloromethane and stir for 5min, let stand to separate the liquid, concentrate the dichloromethane layer under reduced pressure; add 500g of ethyl acetate and 50g of isopropyl to the concentrate Alcohol, stirred and dissolved, crystallized at 20-30°C for 6h, suction filtered, and the filter cake was dried at 55-65°C for 6h, and 123g of hydroxychloroquine was obtained with a purity of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com