Chloroquine phosphate enantiomer crystal form and preparation method thereof
A technology of chloroquine and chloroquine side chains, applied in the field of /- chloroquine phosphate crystals and its preparation, can solve the problems of cumbersome operation and low yield, and achieve the effect of stable and reliable quality and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
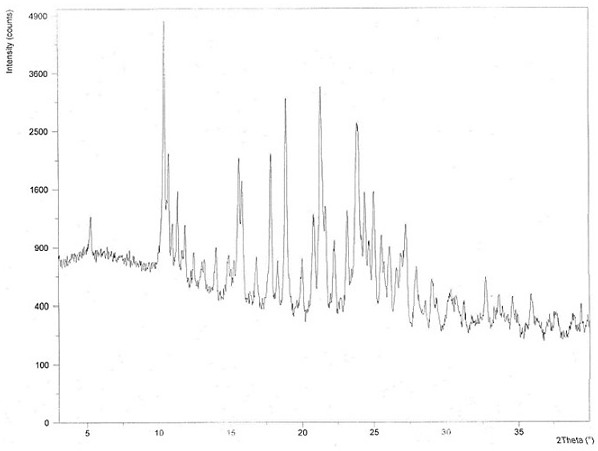
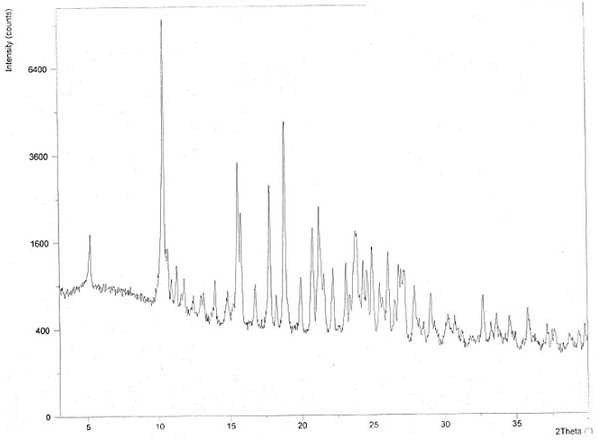
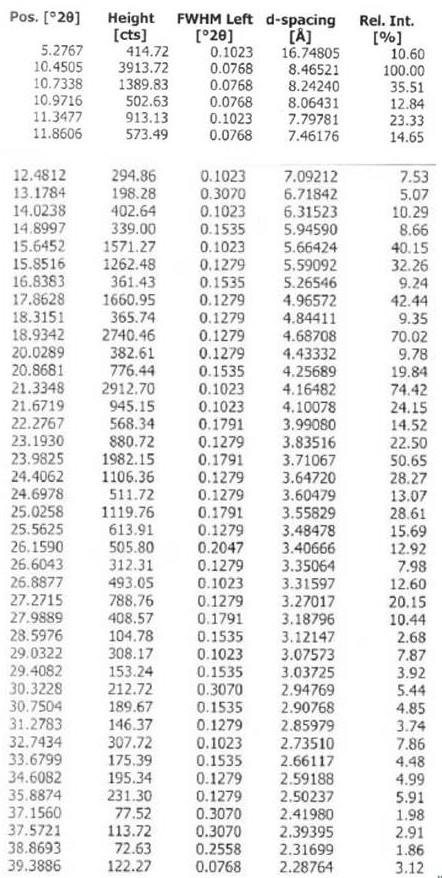
Image
Examples
Embodiment 1
[0068] The preparation of embodiment 1 (S)-chloroquine side chain S-(+)-mandelate
[0069]
[0070] Weigh 72 g (0.48 mol) of chloroquine side chain in a 250 mL round bottom flask, add 150 g of isopropanol and stir to dissolve it; then weigh 36.5 g (0.24 mol) of L-mandelic acid and add to the system, stir at room temperature, A large amount of white solids precipitated; the resulting white solids were filtered by suction and placed in a vacuum oven at 50°C. After drying for 8 hours, 68.8 g of white solid (yield 94.9%) was obtained.
[0071] The measured specific rotation value: S-chloroquine side chain mandelate: =56.5° (c =1.0g / 100mL H 2 O)
Embodiment 2
[0072] The preparation of embodiment 2 S-chloroquine side chain
[0073]
[0074] Dissolve the (S)-chloroquine side chain S-(+)-mandelate prepared in Example 1 with 200 mL of water, add NaOH to adjust the pH to 8-9, add an appropriate amount of sodium chloride to the aqueous phase, and extract with dichloromethane ; Dry over anhydrous sodium sulfate and concentrate to give 35.3 g (1 equivalent, 0.251 mol) of a colorless oil.
Embodiment 3
[0075] The preparation of embodiment 3 (S)-chloroquine phosphate
[0076] All the S-chloroquine side chains prepared in Example 2 were transferred to a three-necked flask, weighed 44.8 g 4,7-dichloroquinoline (0.9 equivalents, 0.226mol), 6 mL Virahol, heated and stirred at 100 ° C . After reacting for 18 h, the raw materials basically reacted completely, stop the reaction, and wait for it to cool down naturally; add NaOH solution to adjust the pH to 12, extract and concentrate with DCM; add dilute HCl solution to adjust the pH to 2, and then adjust the aqueous phase back to alkaline, DCM extraction and concentration gave 72.4 g (HPLC showed product content 75%) yellow oil. Add 200 mL of absolute ethanol to dissolve the oil. Heat to reflux at 80°C for 15min to stabilize the temperature; add 2 equivalents of phosphoric acid (0.34mol, 17.7 mL) dropwise, and stir at 30°C for 1h. A large amount of white solids precipitated, stopped heating, cooled to room temperature; filtered, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com