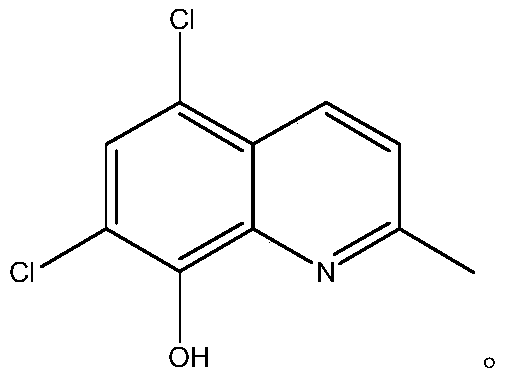
Preparation method of chloroquinaldol
A technology for chloroquinadol and chloroquinadol hydrochloride, applied in the field of medicine, can solve the problems of lack of chloroquinadol product quality research, affecting product stability, efficacy and metabolism, etc., and achieves the effects of easy operation and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
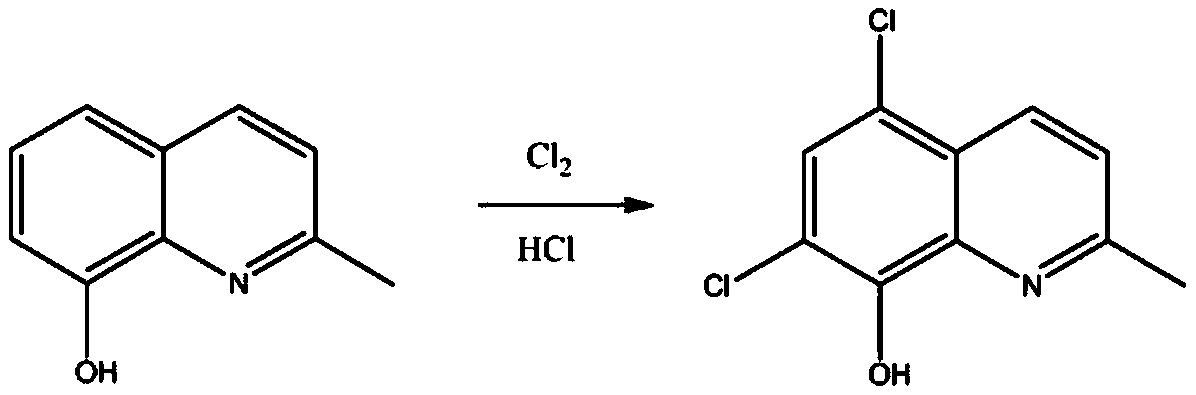
[0035] Under the condition of avoiding light and nitrogen protection, add 200ml of 20wt.% hydrochloric acid, 10 grams of 8-hydroxy-2-methylquinoline into a 500ml four-necked bottle, and feed 9.8 grams of chlorine gas until the chlorination reaction is completed, filter, and the filter cake dissolves. Add 10ml of 0.05M citric acid-sodium dihydrogen phosphate solution in water under the condition of dark and nitrogen protection, adjust the pH to 4.5 with sodium isooctanoate, filter, and refine with ethanol to obtain 14.14 g of chloroquinaldol with a yield of 98.6% , HPLC purity 99.99%.
Embodiment 2
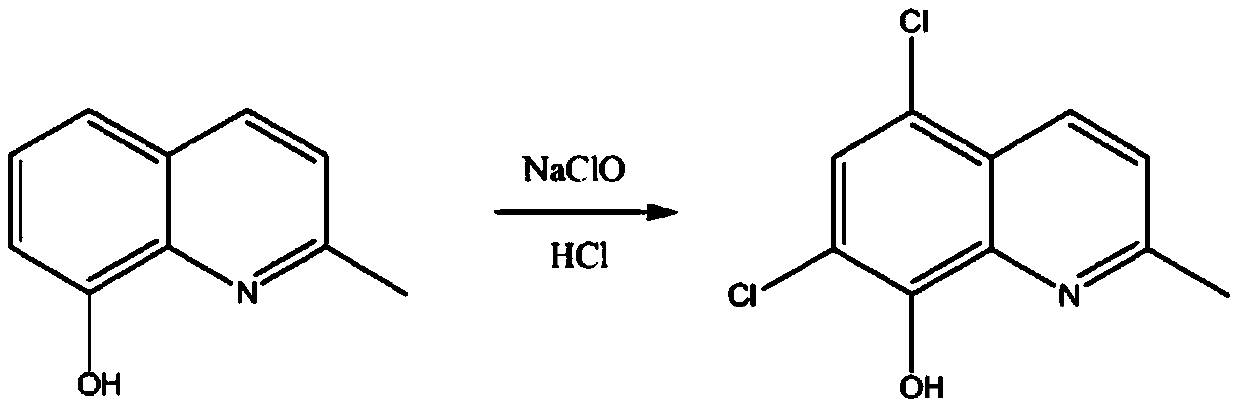
[0037] Under the condition of avoiding light and nitrogen protection, add 200ml of 20wt.% hydrochloric acid, 10 grams of 8-hydroxy-2-methylquinoline to a 500ml four-necked bottle, add dropwise 103 grams of 10wt.% sodium hypochlorite solution, and keep warm until the chlorination reaction Finish, filter, dissolve the filter cake in water, under the condition of dark and nitrogen protection, add 20ml of 0.05 citric acid-sodium dihydrogen phosphate solution, adjust the pH to 4.5 with sodium isooctanoate, filter, and refine with ethanol to obtain 14.18 grams of chloroquinaldol , the yield was 98.9%, and the HPLC purity was 99.99%.
Embodiment 3
[0039] Under the condition of avoiding light and nitrogen protection, add 200ml of formic acid and 10 grams of 8-hydroxy-2-methylquinoline into a 500ml four-necked bottle, feed 9.8 grams of chlorine gas until the chlorination reaction is completed, filter, and the filter cake is dissolved in water. Under the condition of avoiding light and nitrogen protection, add 15ml of 0.05M citric acid-sodium dihydrogen phosphate solution, adjust the pH to 4.5 with sodium isooctanoate, filter, and refine with ethanol to obtain 14.15 grams of chloroquinaldol, with a yield of 98.7%, HPLC purity is 99.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com