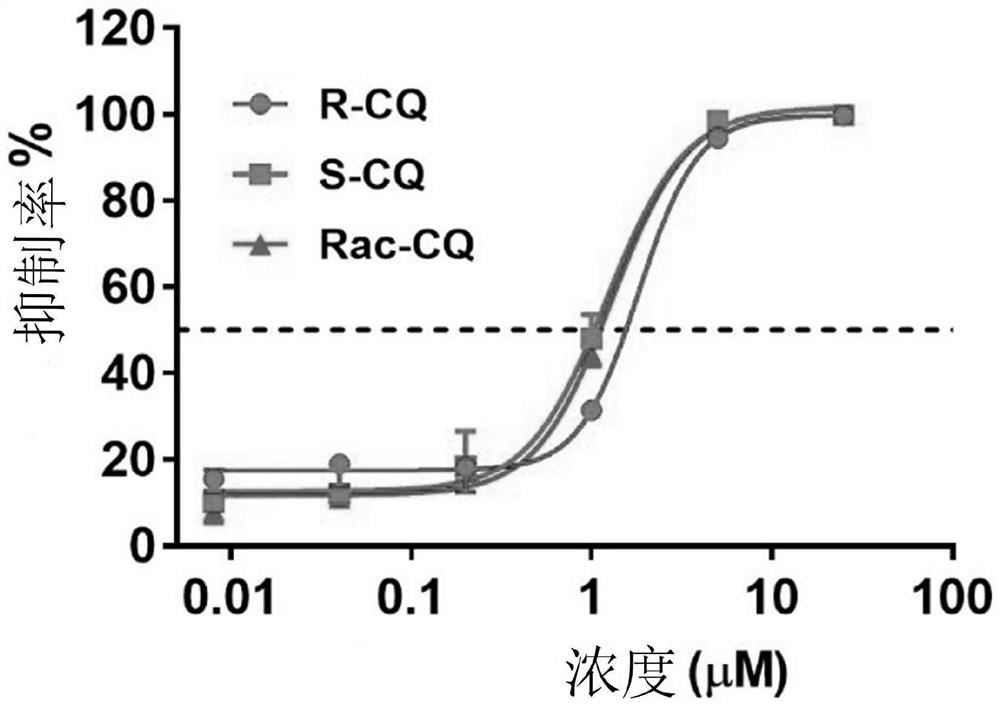
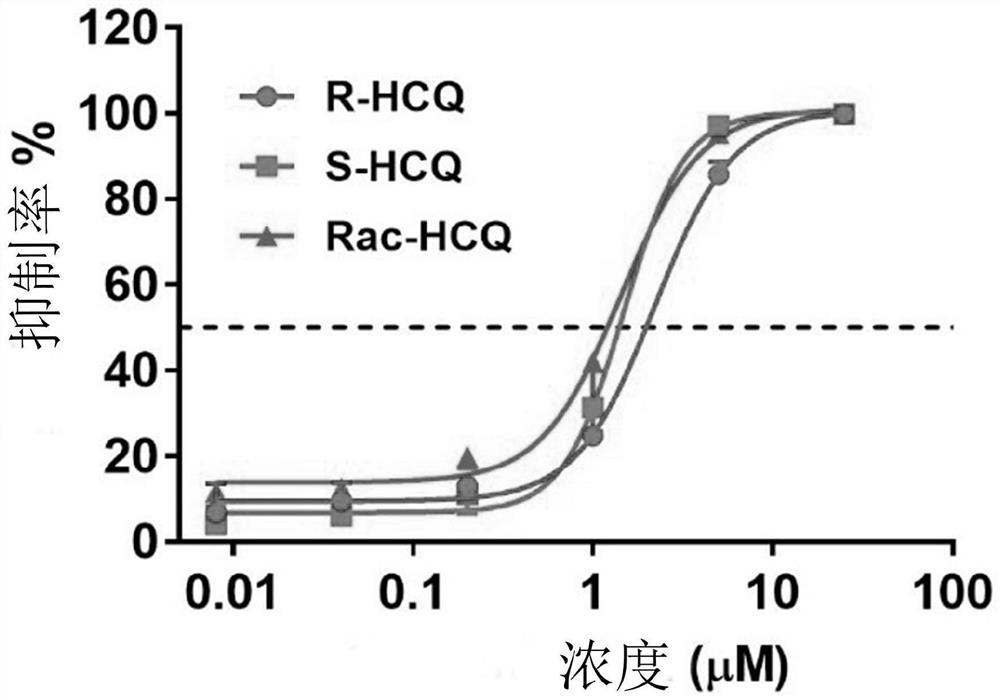
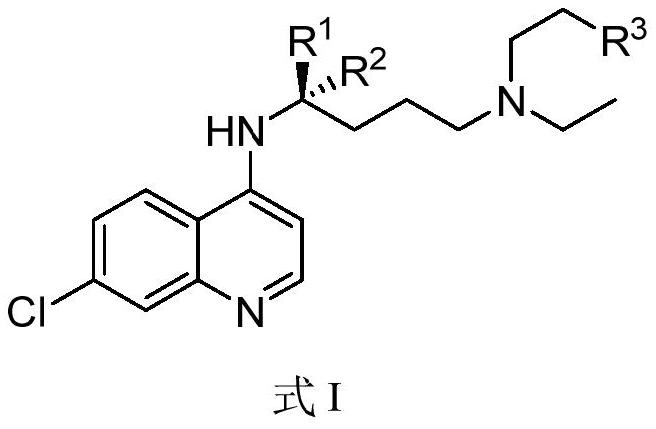
Chiral chloroquine, hydroxychloroquine and derivatives thereof as well as preparation method and application of chiral chloroquine, hydroxychloroquine and derivatives thereof
A compound, polymorphic technology, applied in the field of chiral chloroquine, hydroxychloroquine and derivatives thereof and their preparation, can solve problems such as toxicity not being reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Preparation of Chiral Chloroquine by Chiral High Performance Liquid Chromatography
[0165] The racemic chloroquine phosphate 7 purchased from the market was converted into free racemic chloroquine 8 under alkaline conditions.
[0166]
[0167] At 0°C, 13.0 grams of chloroquine phosphate was dissolved in 75 milliliters of water, followed by the addition of 50 milliliters of 12% NaOH aqueous solution. After stirring for half an hour, 25 milliliters of ethyl acetate was added, and the stirring was continued for half an hour. The reaction solution was naturally raised to room temperature, extracted three times with 100 ml of ethyl acetate, combined the organic phases, washed with 150 ml of saturated brine and water, dried by adding anhydrous sodium sulfate, and filtered to remove sodium sulfate. The organic solvent was removed with a rotary evaporator to obtain 7.6 grams of free chloroquine in the form of light yellow viscous liquid, with a yield of 94%.
[0168] 1 H ...
Embodiment 2
[0172] Preparation of Optically Pure Chloroquine Phosphate
[0173] R and S Free State Chloroquine Converted to Optically Pure Chloroquine Phosphate
[0174]
[0175] Dissolve 640 mg of (S)-chloroquine 9 in 4 mL of ethanol and heat to reflux. 0.25 milliliters of 85% phosphoric acid was added dropwise to the above solution, and the reaction was refluxed for two hours, at which time a large amount of white solids were precipitated. After the reaction solution was cooled to room temperature, it was filtered, and the filter cake was washed three times with 1 milliliter of ethanol to obtain 868 mg of white (S)-chloroquine phosphate 10 solids, with a yield of 84%, [α] D 27.8 =79.7 (c=0.5,H 2 O).
[0176]
[0177] Dissolve 640 mg of (R)-chloroquine 11 in 4 mL of ethanol and heat to reflux. 0.25 ml of 85% phosphoric acid was added dropwise to the above solution, and the reaction was refluxed for two hours, at which time a large amount of white solids were precipitated. The...
Embodiment 3
[0179] Preparation of Chiral Hydroxychloroquine by Chiral High Performance Liquid Chromatography
[0180] Racemic hydroxychloroquine sulfate 1 purchased from the market is converted into free racemic hydroxychloroquine 2 under alkaline conditions.
[0181]
[0182] At 0°C, 10.9 grams of hydroxychloroquine sulfate was dissolved in 75 milliliters of water, then 25 milliliters of 12% NaOH aqueous solution was added, after stirring for half an hour, 25 milliliters of ethyl acetate was added, and stirring was continued for half an hour. The reaction solution was naturally raised to room temperature, extracted three times with 100 ml of ethyl acetate, combined the organic phases, washed with 150 ml of saturated brine and water, dried by adding anhydrous sodium sulfate, and filtered to remove sodium sulfate. The organic solvent was removed with a rotary evaporator to obtain 7.7 grams of free hydroxychloroquine in the form of light yellow viscous liquid, with a yield of 91%.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Quality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com