Preparation process of chloroquine phosphate
A chloroquine phosphate and preparation technology, which is applied in the field of medicine and chemical industry, can solve the problems of being unfriendly to the human body and the environment, low production efficiency, high process energy consumption, etc., and achieve the effects of simple operation, improved production efficiency and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
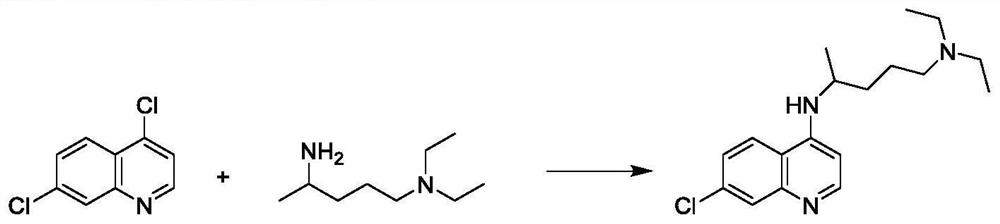
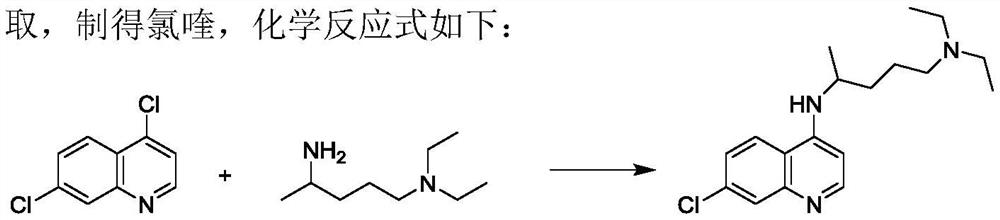
[0042] Add 30 g of 4,7-dichloroquinoline and 24 g of 2-amino-5-diethylaminopentane into a three-necked round-bottomed flask, stir to raise the temperature, keep the reaction at 135°C-140°C for more than 24 hours, and determine the end point of the reaction by HPLC detection. Cool down to about 100°C, add 75g of 6% NaOH solution, measure the pH value of about 11-12, continue to cool down to about 40°C, add 90g of dichloromethane, stir and stand for stratification, collect the organic layer, and use dichloromethane for the water layer 54g was extracted twice, and the organic layers were combined and washed three times with 120g of water. The organic layer was evaporated to dryness in a water bath at 65 °C under reduced pressure, 200 g of isopropyl ether was added, stirred and crystallized at -5 °C to 0 °C for 3 h, and suction filtered to obtain a khaki solid with a wet weight of 43.5 g. Air-dried at 50°C to obtain 35.0 g of dry product with a yield of 72.34%.
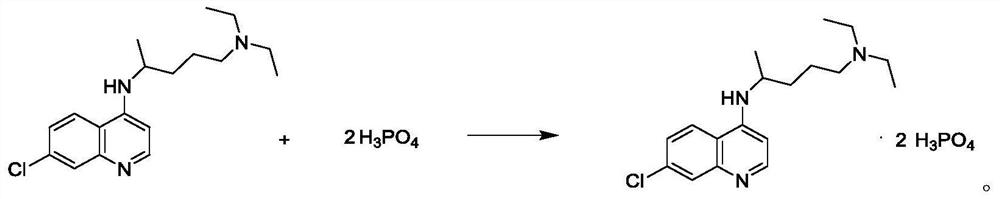
[0043] Salt form...
Embodiment 2
[0046]Condensation: Add 50g of 4,7-dichloroquinoline and 50g of 2-amino-5-diethylaminopentane into a three-neck round-bottomed flask, stir and raise the temperature, keep the temperature at 135℃~140℃ for more than 24h, and judge the end point of the reaction by HPLC detection . Cool down to about 100°C, add 125g of 6% NaOH solution, measure the pH value of about 11-12, continue to cool down to about 40°C, add 150g of dichloromethane, stir and stand for stratification, collect the organic layer, and use dichloromethane for the water layer 90g was extracted twice, and the organic layers were combined and washed three times with 200g of water. The organic layer was evaporated to dryness in a water bath at 65°C under reduced pressure, 200g of isopropyl ether was added, stirred and crystallized at 0-5°C for 3h, and suction filtered to obtain a khaki solid with a wet weight of 73g. Air-dried at 50°C to obtain 58.5 g of dry product with a yield of 72.44%.
[0047] Salt formation: a...
Embodiment 3
[0050] Condensation: Add 50g of 4,7-dichloroquinoline and 60g of 2-amino-5-diethylaminopentane into a three-neck round bottom flask, stir and raise the temperature, keep it at 135℃~140℃ for more than 24h, and judge the end point of the reaction by HPLC detection . Cool down to about 100°C, add 125g of 6% NaOH solution, measure the pH value of about 11-12, continue to cool down to about 40°C, add 150g of dichloromethane, stir and stand for stratification, collect the organic layer, and use dichloromethane for the water layer 90g was extracted twice, and the organic layers were combined and washed three times with 200g of water. The organic layer was evaporated to dryness in a water bath at 65 °C under reduced pressure, 200 g of isopropyl ether was added, stirred and crystallized at -5 °C to 0 °C for 3 h, and suction filtered to obtain a khaki solid with a wet weight of 80 g. Air-dried at 50°C to obtain 64.5 g of dry product with a yield of 79.88%.
[0051] Salt formation: add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com