Hydroxychloroquine sulfate and preparation method thereof
A technology of hydroxychloroquine sulfate and hydroxychloroquine, applied in the field of chemistry or medicinal chemistry, can solve the problems of long production time, cumbersome process, unfriendly environment, etc., and achieve the effects of reducing pollution and consumption, avoiding high pollution, and simple and rapid operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A kind of preparation method of hydroxychloroquine sulfate is characterized in that, comprises the following steps:
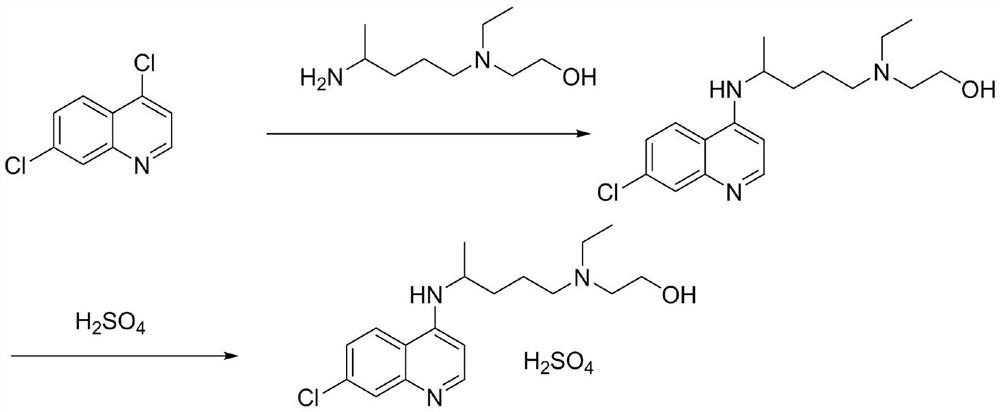
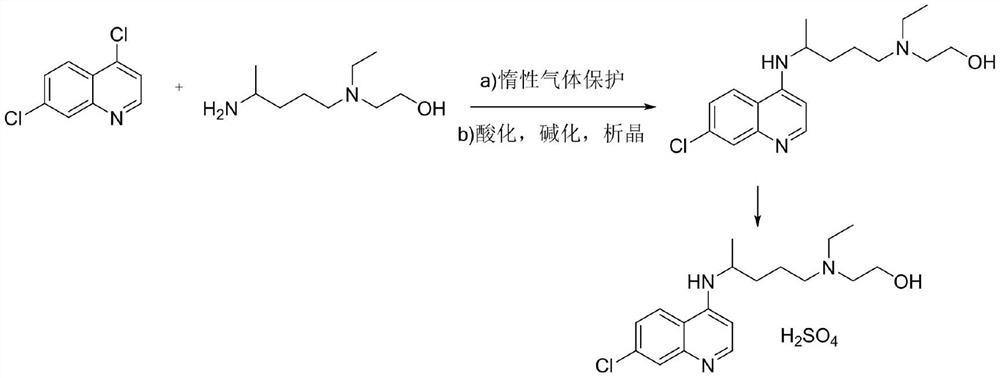
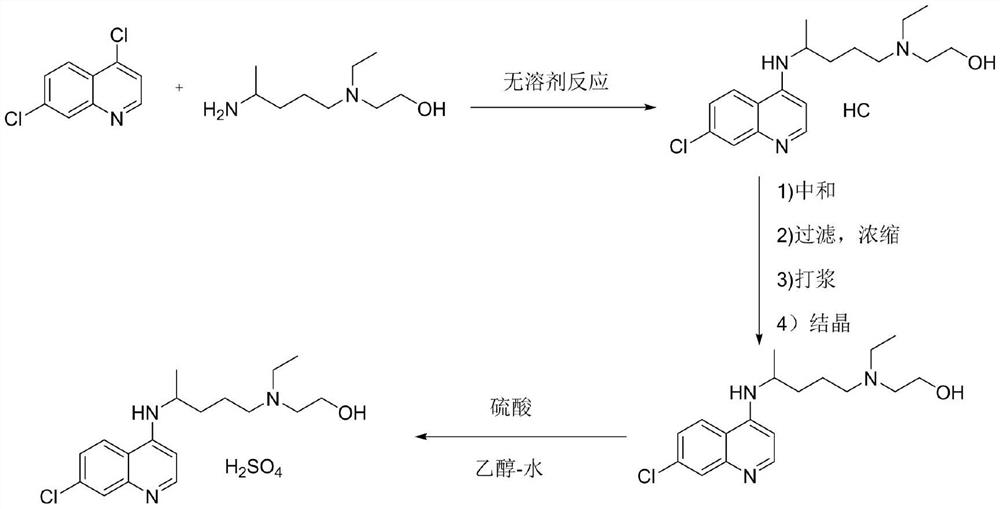
[0037] (1) Synthesis of hydroxychloroquine: after mixing 4,7-dichloroquinoline with hydroxychloroquine side chain (i.e. 5-(N-ethyl-N-2-hydroxyethylamine)-2-pentylamine), in Heat and condense under an organic base catalyst, add water solution, cool down and crystallize to obtain hydroxychloroquine, the HPLC purity of hydroxychloroquine is ≥96.50%, and the maximum simple impurity content is <0.10%. Wherein, the molar ratio of 4,7-dichloroquinoline, hydroxychloroquine side chain, and base catalyst is 1:0.8-1.3:0.8-1.3; the mass ratio of 4,7-dichloroquinoline to water is 1:1-6; the reaction The temperature is 80-130°C, and the reaction time is 13-40h; the organic base catalyst is selected from one or more of triethylamine, ethanolamine, and pyridine; when adding water, keep the temperature not lower than 85°C, and discard the organic base in the lower layer....
Embodiment 1
[0040] (1) Synthesis of Hydroxychloroquine:
[0041] In a 500mL three-necked flask, add 4,7-dichloroquinoline: 100.0g (0.51mol) and hydroxychloroquine side chain: 70.0g, add 0.8eq (0.51mol) of triethylamine (29.8g) and heat up to 80°C, React for 40 hours, HPLC monitors that the content of 4,7-dichloroquinoline is less than 2.5%, add 100g of water, separate the phases, keep the water phase, cool down to room temperature, keep warm for crystallization, drop to 0°C, keep warm for crystallization, suction filter, filter The cake was vacuum-dried to obtain about 144.5 g of hydroxychloroquine, with a yield of 85.20%, an HPLC purity of 96.59%, and a maximum impurity content of 0.08%.
[0042] (2) the synthesis of hydroxychloroquine sulfate:
[0043] Take 50.0g of hydroxychloroquine and add it to a 1000mL four-neck flask, add 50mL of ethyl acetate and 250mL of ethanol, heat up and stir to dissolve, wait until it is completely dissolved, add 11.7g of concentrated sulfuric acid dropwis...
Embodiment 2
[0045] (1) Synthesis of Hydroxychloroquine:
[0046]In a 500mL three-necked flask, add 4,7-dichloroquinoline: 100.0g (0.51mol) and hydroxychloroquine side chain: 79.0g, add 0.9eq of ethanolamine (28.3g) and raise the temperature to 90°C, react for 30h, and monitor by HPLC to The content of 4,7-dichloroquinoline is less than 2.5%, add 200g of water, separate the phases, keep the water phase, cool down to room temperature, keep warm for crystallization, drop to 2°C, keep warm for crystallization, filter with suction, and dry the filter cake in vacuum to obtain hydroxyl Chlorine is about 146.4g, the yield is 86.32%, the HPLC purity is 97.24%, and the maximum simple impurity content is 0.07%.
[0047] (2) the synthesis of hydroxychloroquine sulfate:
[0048] Take 50.0g of hydroxychloroquine refined product and add it to a 1000mL four-neck flask, add 100mL ethyl acetate and 300mL ethanol, heat up and stir to dissolve, wait until it is completely dissolved, add 13.2g of concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com