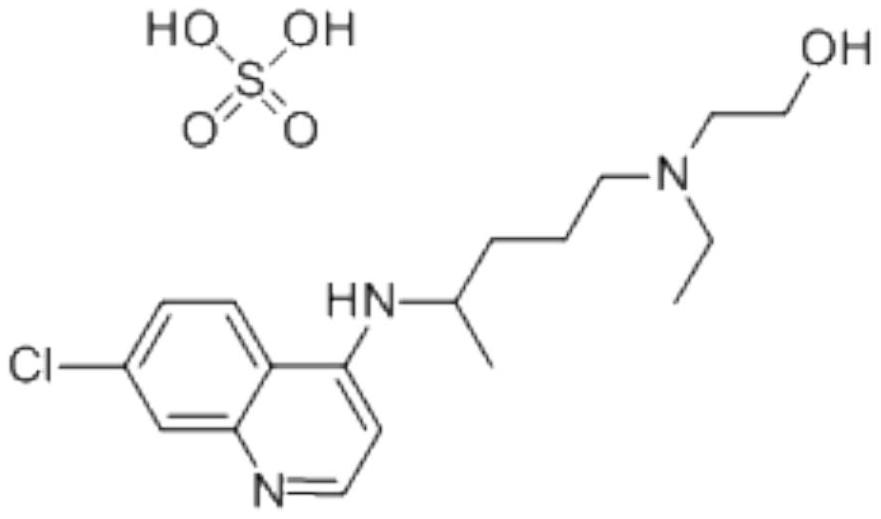
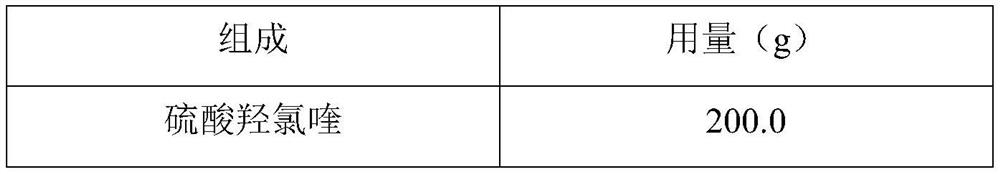
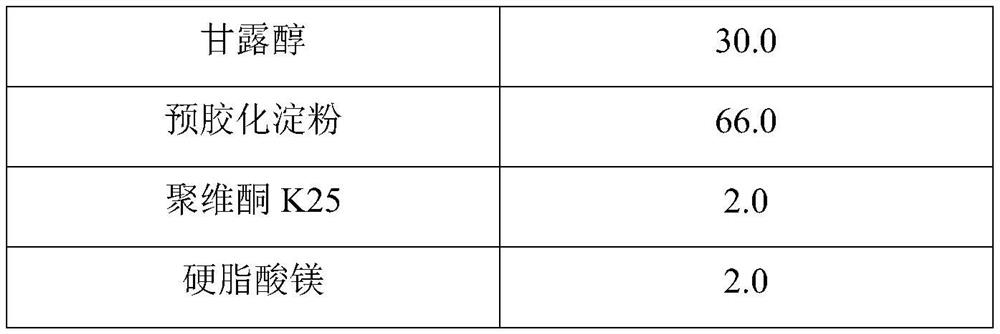
Hydroxychloroquine sulfate pharmaceutical preparation
A technology for hydroxychloroquine sulfate and pharmaceutical preparations, applied in the field of medicine, can solve the problems of tablet fragility, cracking, sticking and punching, etc., achieve good dissolution properties, and solve the effects of poor compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033]
[0034] Preparation:
[0035] (1) Premixing: add mannitol, pregelatinized starch, hydroxychloroquine sulfate and povidone K25 into the wet mixing granulator and mix, turn on the stirring paddle (50-70rpm) and the cutting knife (250-500rpm), Mix for 600 seconds, and pass the resulting mixed powder through a swinging granulator (screen aperture: 40 mesh) once to obtain hydroxychloroquine sulfate tablet premixed powder;
[0036] (2) Granulation: with water as the wetting agent, the atomization pressure is set and controlled at 0.3MPa, the hydroxychloroquine sulfate tablet premixed powder is granulated, and after the liquid spray is finished, it is dried, and the granules are passed through a swinging granulator (sieve) Mesh pore size: 26 meshes) whole grains to obtain solid granules containing hydroxychloroquine sulfate;
[0037] (3) Blending and tableting: uniformly mix the solid granules containing hydroxychloroquine sulfate and magnesium stearate to obt...
Embodiment 2
[0039] composition Dosage (g) Hydroxychloroquine Sulfate 200.0 Mannitol 46.0 pregelatinized starch 140.0 gelatin 6.0 magnesium lauryl sulfate 8.0
[0040] Preparation:
[0041] (1) Premixing: add mannitol, pregelatinized starch, hydroxychloroquine sulfate and gelatin into the wet mixing granulator and mix, turn on the stirring paddle (50-70rpm) and cutter (250-500rpm), and mix for 600 seconds , the resulting mixed powder is passed through a oscillating granulator (screen aperture: 40 mesh) once to obtain hydroxychloroquine sulfate tablet premixed powder;
[0042] (2) Granulation: with water as the wetting agent, the atomization pressure is set and controlled at 0.2MPa, the hydroxychloroquine sulfate tablet premixed powder is granulated, and after the liquid spray is finished, it is dried, and the granules pass through a swinging granulator (sieve) Mesh aperture: 20 mesh) whole grain, obtain the solid grain containing hydroxychloroq...
Embodiment 3
[0045]
[0046]
[0047] Preparation:
[0048] (1) Premixing: add mannitol, pregelatinized starch, hydroxychloroquine sulfate and hypromellose to the wet mixing granulator and mix, turn on the stirring blade (50-70rpm) and the cutting knife (250-500rpm) , mixed for 600 seconds, and the resulting mixed powder was passed through a swinging granulator (screen aperture: 40 mesh) once to obtain hydroxychloroquine sulfate tablet premixed powder;
[0049] (2) Granulation: with water as the wetting agent, the atomization pressure is set and controlled at 0.2MPa, the hydroxychloroquine sulfate tablet premixed powder is granulated, and after the liquid spray is finished, it is dried, and the granules pass through a swinging granulator (sieve) Mesh aperture: 18 meshes) whole grains, to obtain solid particles containing hydroxychloroquine sulfate;
[0050] (3) Blending and tableting: uniformly mix the solid granules containing hydroxychloroquine sulfate and magnesium stearate to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com