Clopidogrel hydrogen sulfate tablet and preparation method thereof
A technology of clopidogrel bisulfate tablets and clopidogrel bisulfate is applied in the directions of pill delivery, blood diseases, extracellular fluid diseases, etc. and other problems, to achieve the effect of easy industrialization promotion, reducing sticking and flushing, and solving easy sticking and flushing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
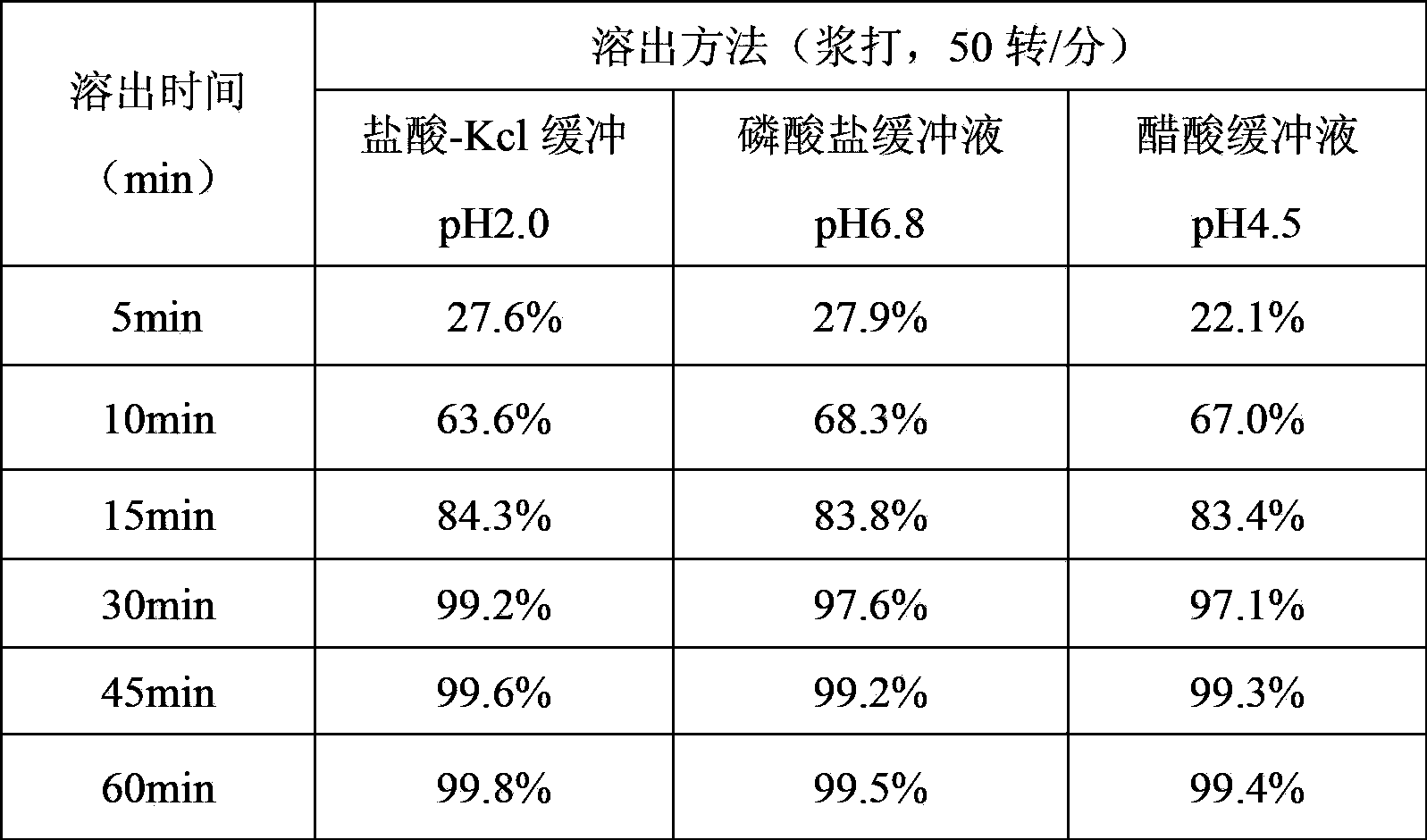
[0019] The clopidogrel bisulfate tablet of this embodiment comprises the following components by mass: 39.44 parts of clopidogrel bisulfate, 31.53 parts of lactose, 15 parts of pregelatinized starch, 10 parts of microcrystalline cellulose, 6000 parts of polyethylene glycol 2.7 parts, 1.33 parts of hydrogenated castor oil, 3 parts of coating film.
[0020] The preparation method of the clopidogrel bisulfate tablet provided in this example (the weight of the tablet core is 248 mg, taking the preparation of 1000 tablets as an example), comprises the following steps:
[0021] 1) Dry microcrystalline cellulose and pregelatinized starch at 80°C until the water content is <3% and <14% respectively; then pass clopidogrel bisulfate through a 40-mesh sieve, polyethylene glycol 6000 through a 100-mesh sieve, spare;
[0022] 2) Mix 97.8g of clopidogrel bisulfate, 78.204g of lactose, 24.8g of microcrystalline cellulose, 12.4g of pregelatinized starch and 3.348g of polyethylene glycol 6000...
Embodiment 2
[0028] The clopidogrel bisulfate tablet of this embodiment comprises the following components by mass: 39.44 parts of clopidogrel bisulfate, 31.53 parts of lactose, 15 parts of pregelatinized starch, 10 parts of microcrystalline cellulose, 6000 parts of polyethylene glycol 2.7 parts, 1.33 parts of hydrogenated castor oil, 3 parts of coating film.
[0029] The preparation method of the clopidogrel bisulfate tablet provided in this example (the weight of the tablet core is 248 mg, taking the preparation of 1000 tablets as an example), comprises the following steps:
[0030] 1) Dry microcrystalline cellulose and pregelatinized starch at 80°C until the water content is <3% and <14% respectively; then pass clopidogrel bisulfate through a 40-mesh sieve, polyethylene glycol 6000 through a 100-mesh sieve, spare;
[0031] 2) Mix 97.8g of clopidogrel bisulfate, 78.204g of lactose, 24.8g of microcrystalline cellulose, 12.4g of pregelatinized starch and 3.348g of polyethylene glycol 6000...
Embodiment 3
[0037] The clopidogrel bisulfate tablet of this embodiment comprises the following components by mass: 39.44 parts of clopidogrel bisulfate, 31.53 parts of lactose, 15 parts of pregelatinized starch, 10 parts of microcrystalline cellulose, 6000 parts of polyethylene glycol 2.7 parts, 1.33 parts of hydrogenated castor oil, 3 parts of coating film.
[0038] The preparation method of the clopidogrel bisulfate tablet provided in this example (the weight of the tablet core is 248 mg, taking the preparation of 1000 tablets as an example), comprises the following steps:
[0039] 1) Dry microcrystalline cellulose and pregelatinized starch at 80°C until the water content is <3% and <14% respectively; then pass clopidogrel bisulfate through a 40-mesh sieve, polyethylene glycol 6000 through a 100-mesh sieve, spare;
[0040] 2) Mix 97.8g of clopidogrel bisulfate, 78.204g of lactose, 24.8g of microcrystalline cellulose, 12.4g of pregelatinized starch and 60003.348g of polyethylene glycol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com