Nerve cell protective agent and application thereof in epilepsy prevention and treatment
A technology of nerve cells and cells, applied in the prevention and treatment of epilepsy, in the field of oral tablets, can solve the problems of easy sticking and punching of tablets, and achieve the effect of solving easy sticking and punching, increasing dependence, significant social and economic significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of ethambutol hydrochloride-sodium valproate tablet
[0025] (1) Weigh 10 g of ethambutol hydrochloride, add 200 mL of water to prepare a solution, then add 12 g of kaolin, continue to stir to make it fully adsorbed and mix evenly, dry at 50 ° C at low temperature, and pass through a 20-mesh sieve to obtain ethylamine hydrochloride Butanol-containing granules;
[0026] (2) Take sodium valproate 125g, add in 2L75% ethanol solution and be mixed with solution, then add kaolin 180g, keep stirring to make it fully adsorb and mix evenly, dry, cross 20 mesh sieves, obtain sodium valproate drug-containing granules;
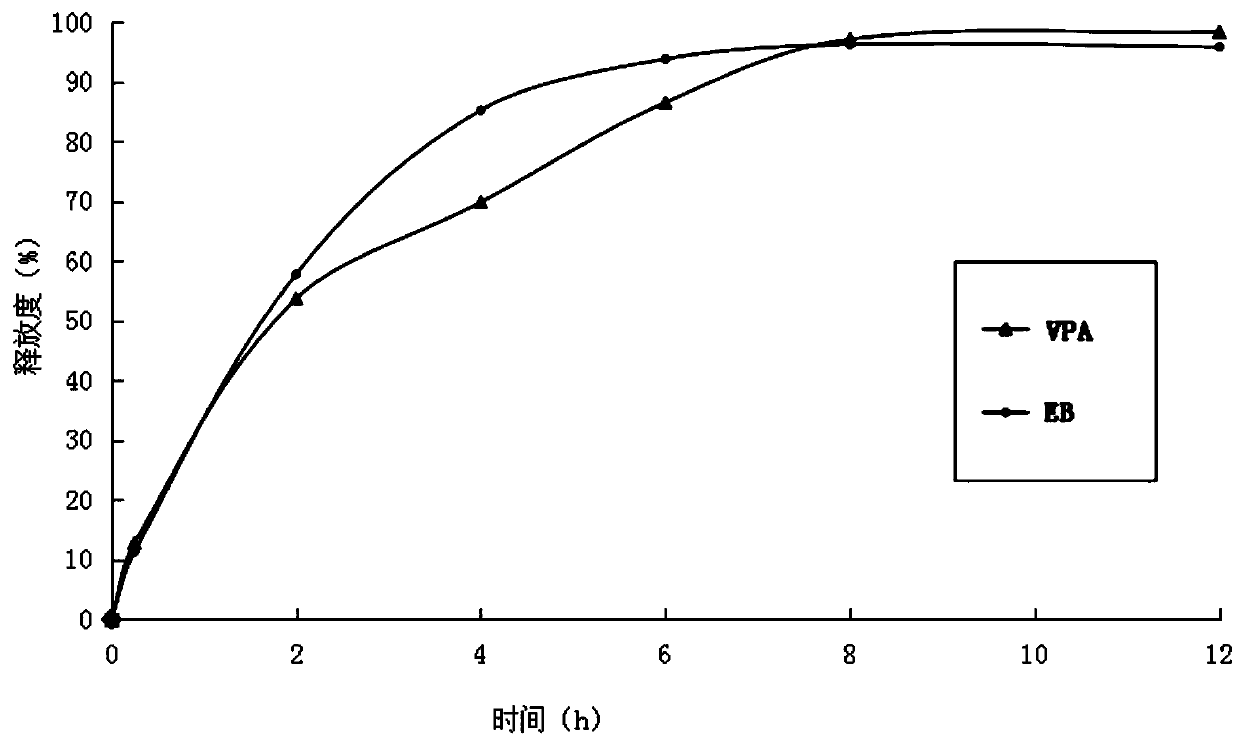
[0027] (3) Mix ethambutol hydrochloride drug-containing granules, sodium valproate drug-containing granules with 50 g of calcium hydrogen phosphate, 20 g of sodium carboxymethyl starch and 3.2 g of magnesium stearate, and directly compress into tablets. During the tableting process, there is no sticking phenomenon. Measure the relea...
Embodiment 2
[0028] Embodiment 2: the preparation of ethambutol hydrochloride-sodium valproate tablet
[0029](1) Weigh 12.5g of ethambutol hydrochloride, add 250mL water to prepare a solution, then add kaolin 15g, keep stirring to make it fully adsorb and mix evenly, dry at 50°C at low temperature, and pass through a 20-mesh sieve to obtain ethanol hydrochloride Ambutol-containing granules;
[0030] (2) Take sodium valproate 125g, add in 2L75% ethanol solution and be mixed with solution, then add kaolin 200g, keep stirring to make it fully adsorb and mix evenly, dry, cross 20 mesh sieves, obtain sodium valproate drug-containing granules;
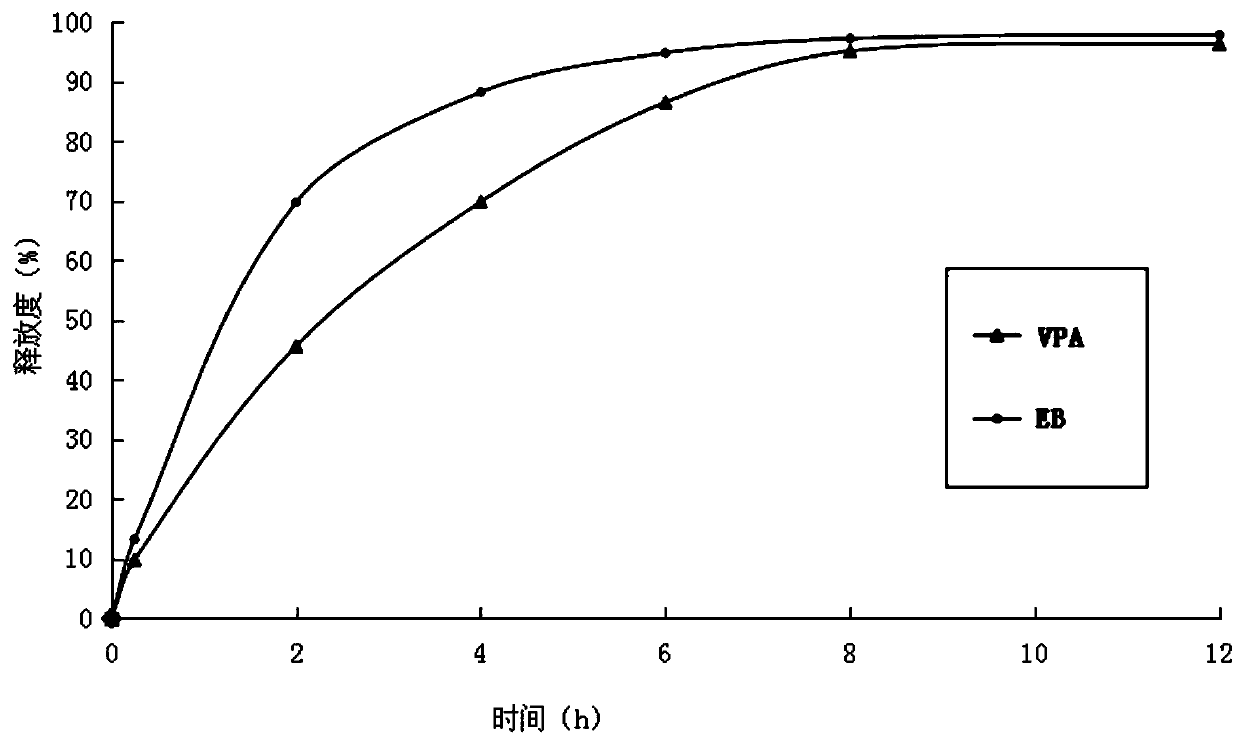
[0031] (3) Mix ethambutol hydrochloride drug-containing granules, sodium valproate drug-containing granules with 60 g of calcium hydrogen phosphate, 25 g of sodium carboxymethyl starch and 3.5 g of magnesium stearate, and directly compress into tablets. During the tableting process, there is no sticking phenomenon. Measure the release rate of medicin...
Embodiment 3
[0032] Embodiment 3: the preparation of ethambutol hydrochloride-sodium valproate tablet
[0033] (1) Weigh 15g of ethambutol hydrochloride, add 300mL of water to prepare a solution, then add 15g of kaolin, keep stirring to make it fully adsorbed and mix evenly, dry at 50°C at low temperature, and pass through a 20-mesh sieve to obtain ethylamine hydrochloride Butanol-containing granules;
[0034] (2) Take sodium valproate 125g, add in 2L75% ethanol solution and be mixed with solution, then add kaolin 200g, keep stirring to make it fully adsorb and mix evenly, dry, cross 20 mesh sieves, obtain sodium valproate drug-containing granules;
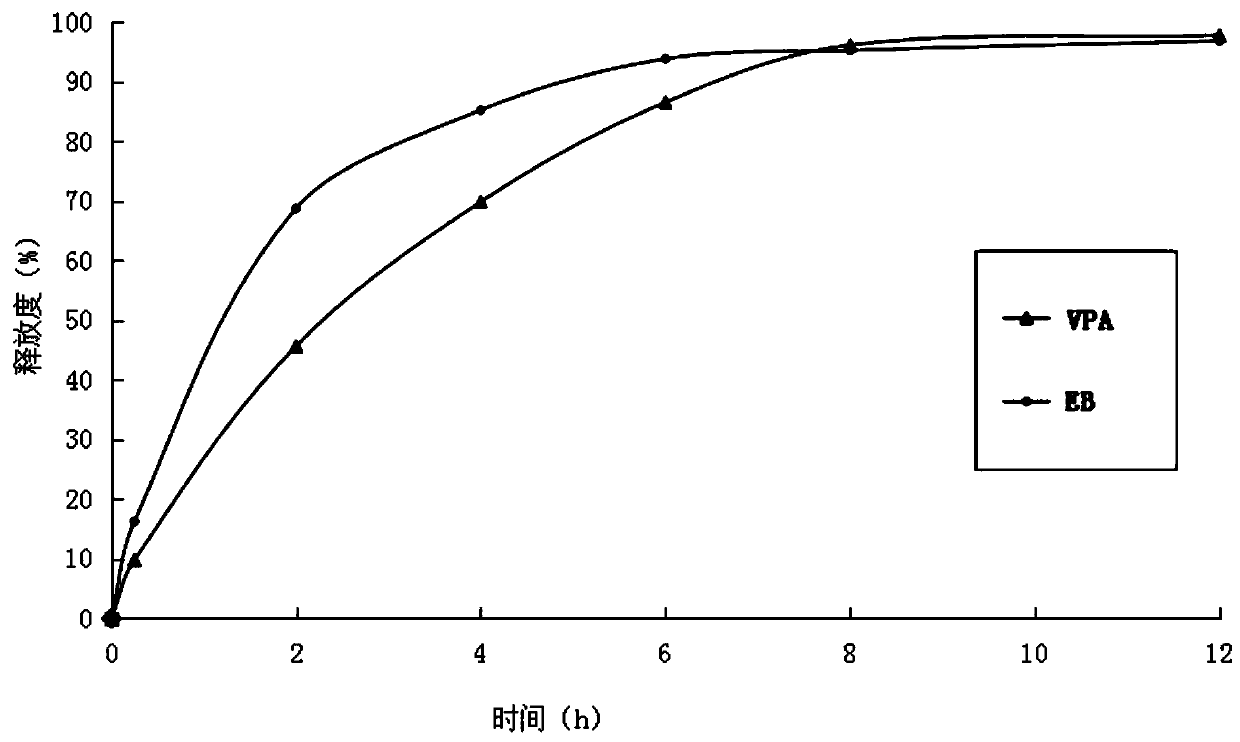
[0035] (3) Mix ethambutol hydrochloride drug-containing granules, sodium valproate drug-containing granules with 60 g of calcium hydrogen phosphate, 25 g of carboxymethyl starch sodium and 3.8 g of magnesium stearate, and directly compress into tablets. During the tableting process, there is no sticking phenomenon. Measure the release rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com