Patents
Literature
1158 results about "Ethylamines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivatives of ethylamine (the structural formula CH3CH2NH2).
In-situ atomic layer deposition
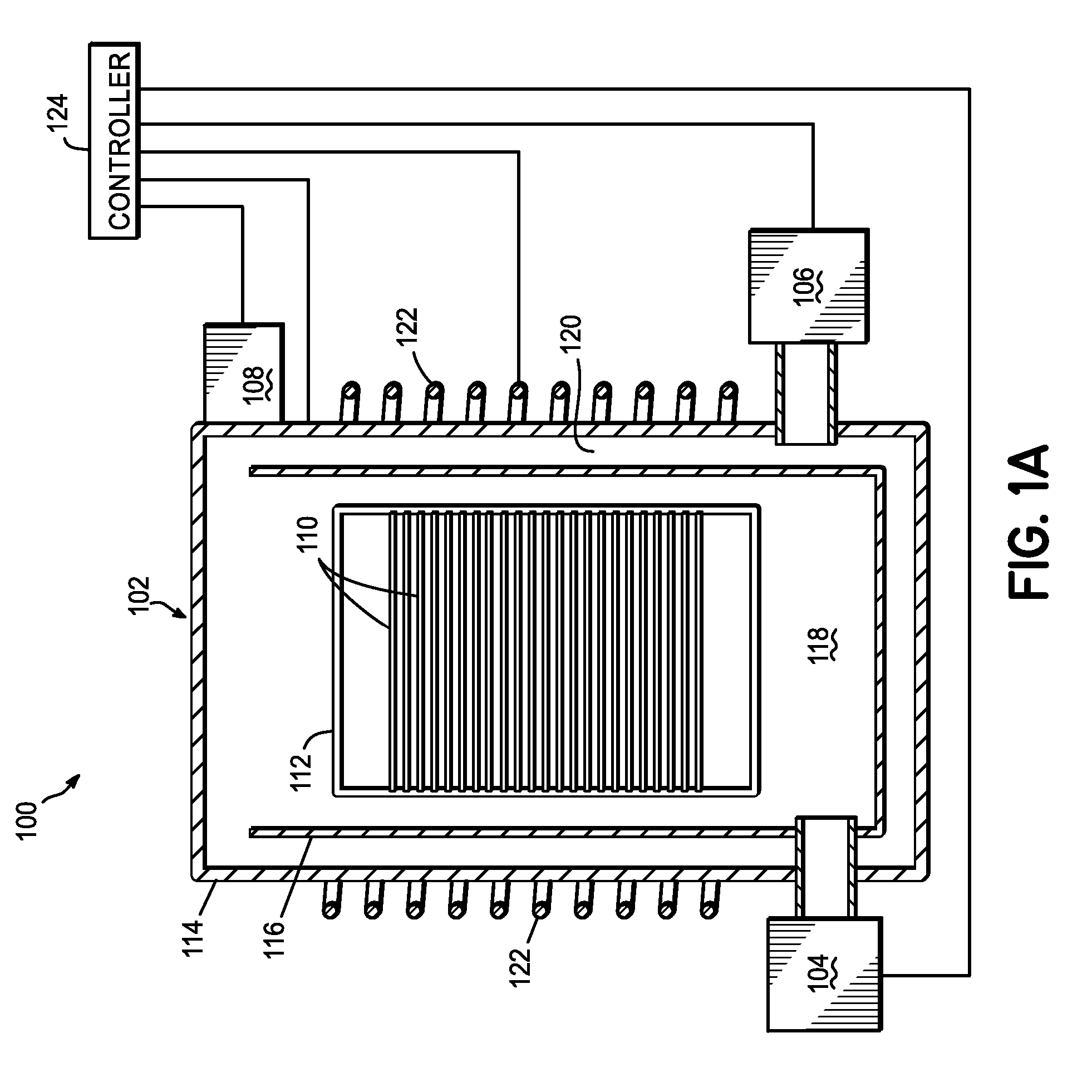
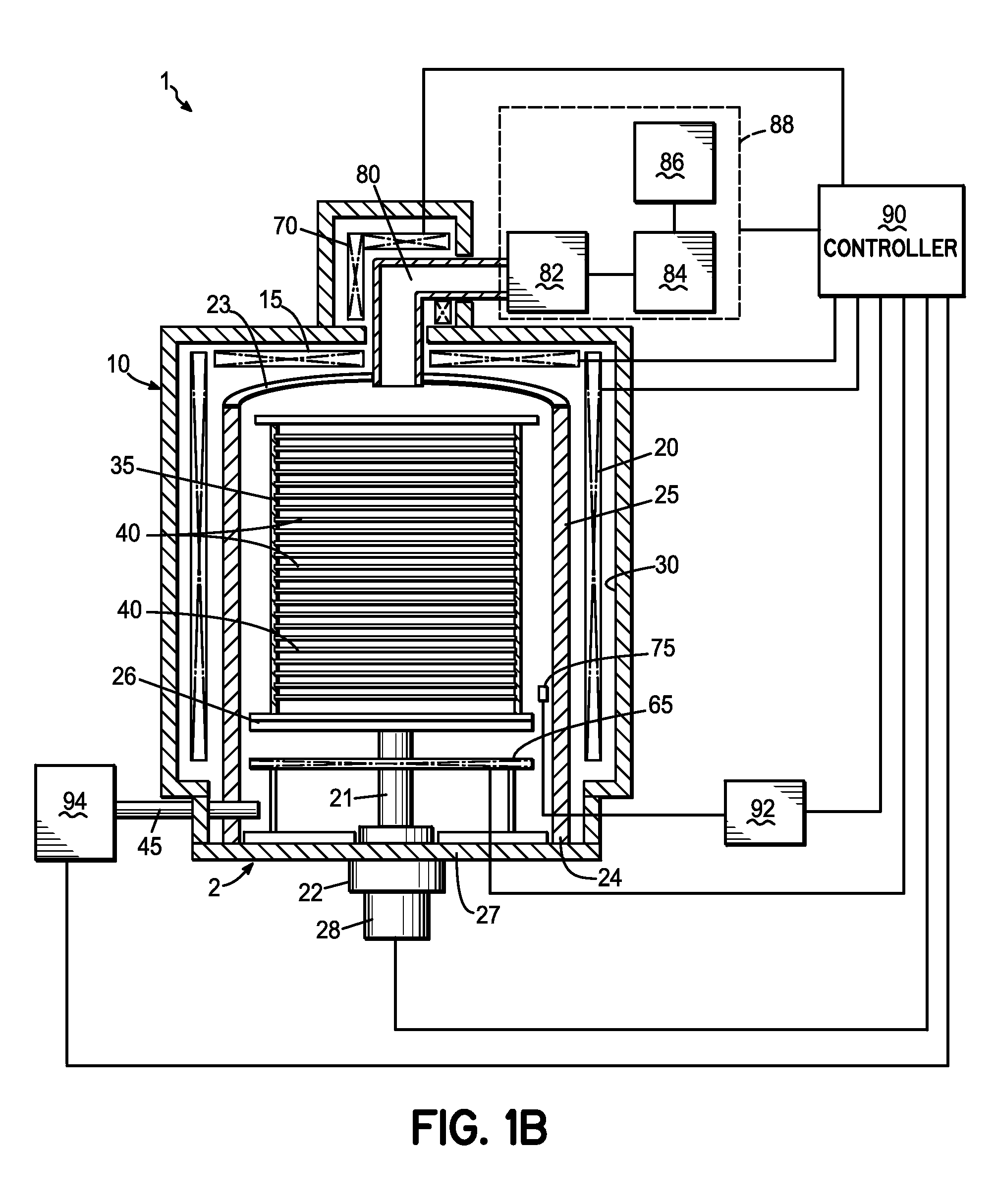
InactiveUS20070037412A1Improve uniformityImprove electrical performanceSemiconductor/solid-state device manufacturingChemical vapor deposition coatingWaferingHafnium
An in situ method for forming a HfO2 high-k dielectric layer in a batch wafer processing system. The method comprises first loading a plurality of wafers into a process chamber, and then pre-treating the plurality of wafers in the process chamber with a first oxidizer. After pre-treating the wafers, and without removing the wafers from the process chamber, the method then comprises depositing HfO2 on the plurality of wafers by atomic layer deposition, which comprises a plurality of deposition cycles, each cycle comprising alternating exposure of the plurality of wafers in the process chamber to a second oxidizer and a hafnium precursor. The hafnium precursor is selected from hafnium tert-butoxide (HTB) or hafnium tetra-diethylamide (TDEAH).
Owner:TOKYO ELECTRON LTD
Composition Containing Statins and Omega-3 Fatty Acids
InactiveUS20080089876A1Hydroxy compound active ingredientsPeptide/protein ingredientsFatty acidStatine
A combination is described comprising at least one omega-3 fatty acid, optionally esterified or salified, at least one statin, Coenzyme Q10, resveratrol, at least one policosanol, pantethine, selenium, and zinc. This combination is endowed with a synergistic effect and is useful in the treatment of disease forms due to insulin resistance and in cardiovascular diseases.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Zirconium oxide fabrics prepared from organic polyzirconium fore spinning solution by silk process
InactiveCN1584155AGood effectRaw materials are cheap and easy to getInorganic material artificial filamentsFuranSolvent
The manufacture method of a kind of zirconia fibre cotton relates to the field of resistance of fire fibre material. We compose van-style acetamide acetone zirconium polymer according to chloridze zirconia, acetamide acetone and three-ethylamine. We use methanol as the dilute impregnant, we mix round them to let hloridize zirconia, acetamide acetone and three-ethylamine react at 40-20 deg.C, and we can receive poly-acetamide acetone zirconium van-substance. Four-hydrogen furan filtrate and remove the outgrowth hydrochloric three-ethylamine. The offspring dissolve in the filature liquid made of methanol. After centrifugal swing, we can receive van-substance fibre. After we do special hear treatment, the zirconia fibre cotton we receive has the characteristic of good filature ability, high content of zirconium, equality andclarity and jarles capability. It can be used as industrial stove, roomage fusion stove, atomic energy reactor and the high temperature heat insulation material used in aviation and military. The material of the invention cost low, the method is easy, the impregnant can be recycled, the preparation of fibre cost low.
Owner:绍兴市圣诺超高温晶体纤维材料有限公司
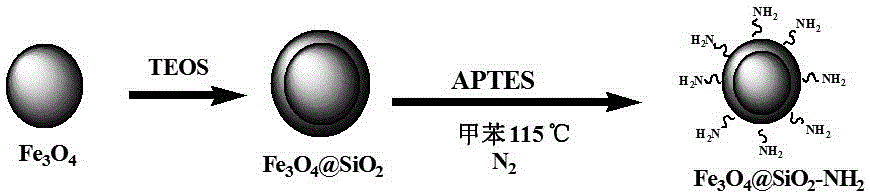
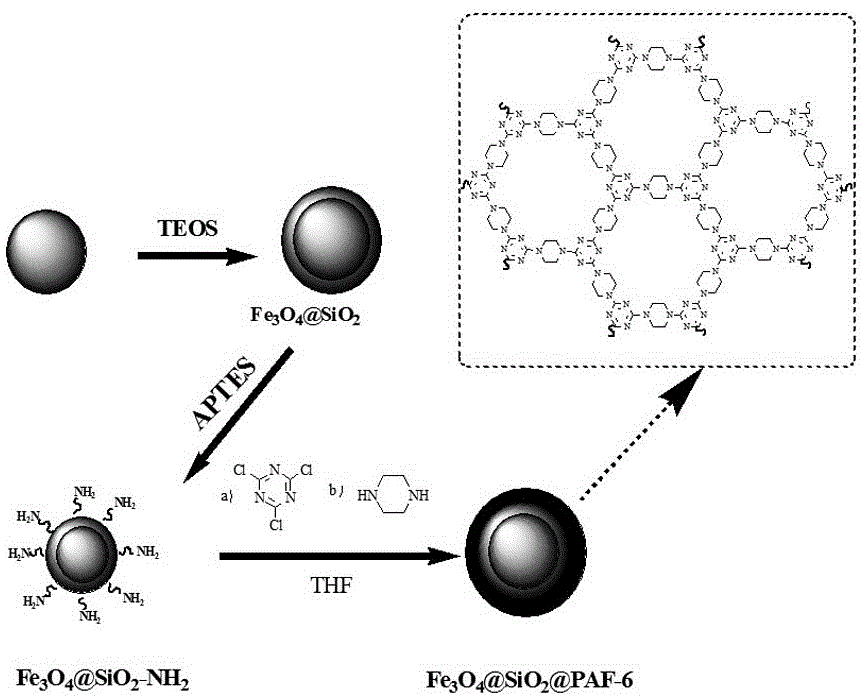
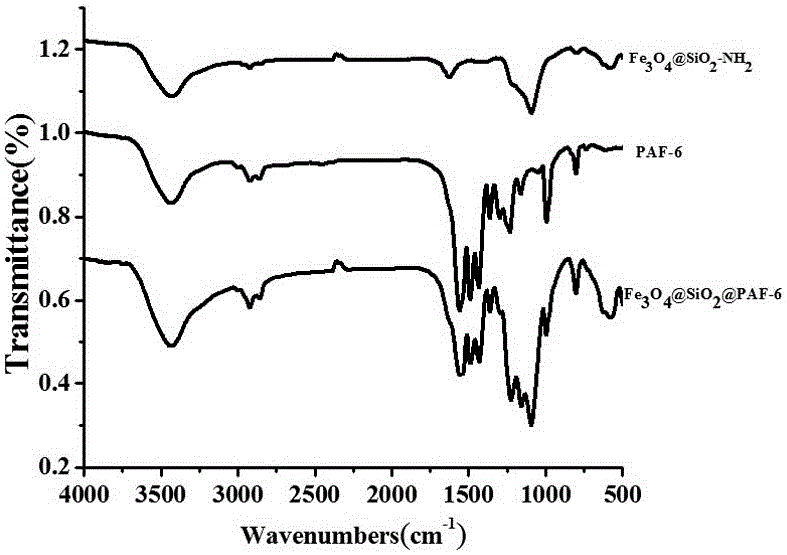
Magnetic PAFs solid-phase extracting agent and preparation method and application thereof
ActiveCN105879842AEasy to separateAvoid inconvenienceOther chemical processesWater contaminantsSilanesCarboxylic acid
The invention discloses a magnetic PAFs solid-phase extracting agent. A preparation method of the magnetic PAFs solid-phase extracting agent includes allowing 3-aminopropyl triethoxy silylation ferroferric oxide to have a temperature-programmed reaction with piperazine and cyanuric chloride in the presence of N, N-diisopropylethylamine to obtain the magnetic PAFs solid-phase extracting agent. The magnetic PAFs solid-phase extracting agent and the preparation method thereof have the advantages that the prepared magnetic PAFs solid-phase extracting agent is good in dispersity and stable in core-shell structure; the preparation method is simple, low in cost, widely applicable, capable of achieving repeated material recycling, and the like; a covalent organic framework bonded to the ferroferric oxide can provide various action sites such as an inclusion interaction site, a hydrogen-bond interaction site, a pi-pi interaction site and an anion exchange site, so that the covalent organic framework has specific recognition and retention acting force on polar substances such as phenols and carboxylic acids.
Owner:ZHENGZHOU UNIV
Ester type fire-resistant hydraulic fluid and preparation method thereof
InactiveCN103013635AInhibition of catalytic oxidationImprove stabilityLubricant compositionPhosphoric acidTriazole derivatives
The invention relates to an ester type fire-resistant hydraulic fluid. The hydraulic fluid comprises the following raw materials by weight percent: 95-99% of base oil, 1.0-5.0% of diphenol propane, 0.005% of dimethylsilicone fluid or dimethylsilicone grease, 0.05% of tricresyl phosphate, 0.1% of benzotriazole, 100 parts per million (PPM) demulsifying agent T1001 or LZ5957 and 0.2-0.3% of triazole derivative, thiadiazole derivative, N-salicylidene ethylamine, N, N'-bis(salicylidene)ethylenediamine, N, N'-bis(salicylidene)propylene diamine or ethylenediamine tetraacetic acid. A preparation method of the ester type fire-resistant hydraulic fluid comprises the following steps of sufficiently and evenly stirring various materials at room temperature according to a formula, and then filtering. The ester type fire-resistant hydraulic fluid has the beneficial effects that the high temperature use performance of the product is greatly improved, the service life of a hydraulic system and the oil changing period of oils are prolonged, and the ester type fire-resistant hydraulic fluid has a good flame-retardant effect and is safe to use. The preparation method has the advantages that operation is convenient, technology and equipment are simple, energy consumption is low, cost is low, and the like.
Owner:ANLU AOSEN PETROCHEM
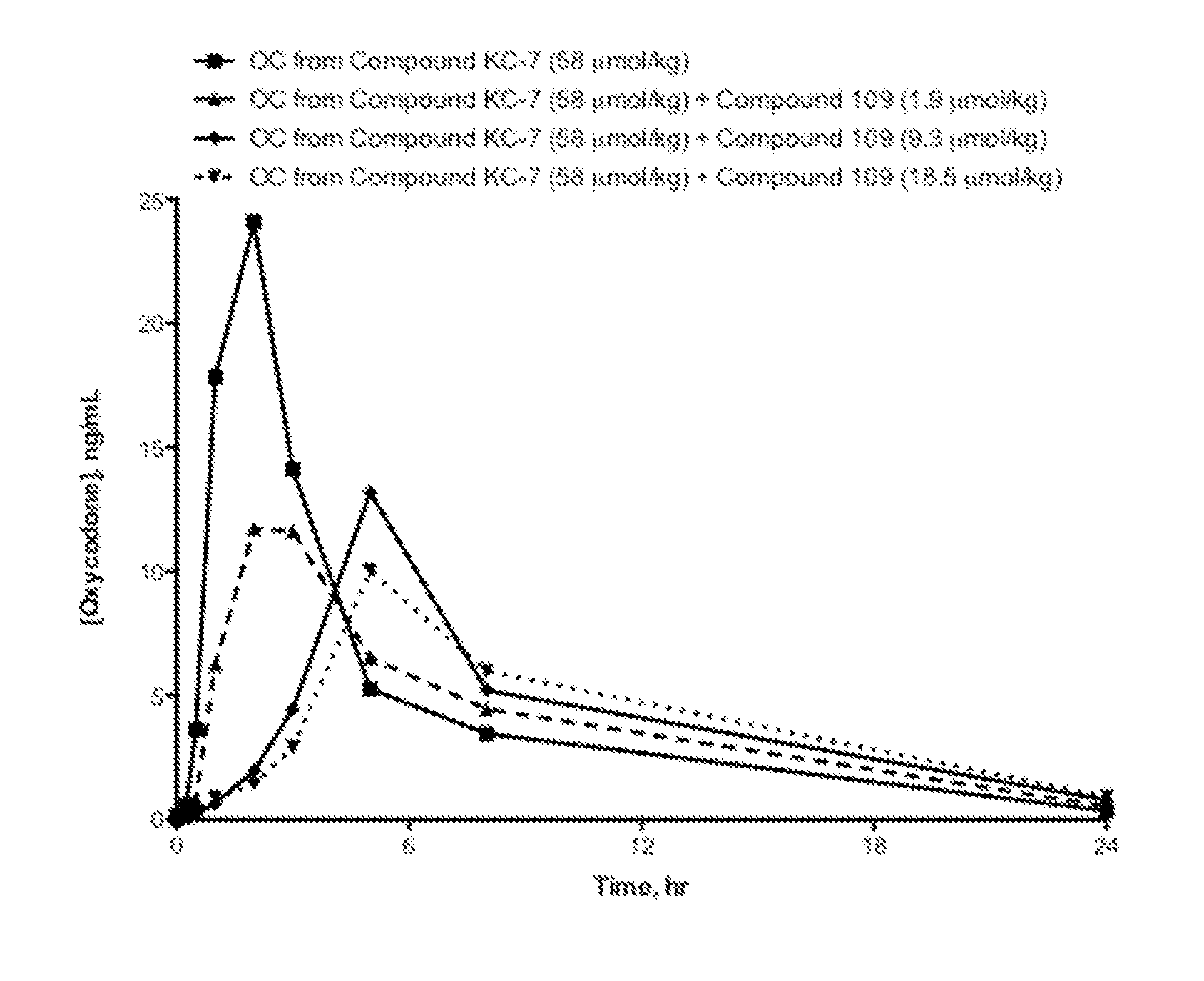
Compositions Comprising Enzyme-Cleavable Oxycodone Prodrug
ActiveUS20120178773A1Efficiently deliverEfficient deliveryBiocideNervous disorderStereochemistryTrypsin inhibitor
The embodiments provide Compound KC-7, N-1-[(S)-2-(oxycodone-6-enol-carbonyl-methyl-amino)-2-carbonyl-sarcosine-ethyl amine]-arginine-glycine-acetate, or acceptable salts, solvates, and hydrates thereof. The present disclosure also provides compositions, and their methods of use, where the \compositions comprise a prodrug, Compound KC-7, that provides controlled release of oxycodone. Such compositions can optionally provide a trypsin inhibitor that interacts with the enzyme that mediates the controlled release of oxycodone from the prodrug so as to attenuate enzymatic cleavage of the prodrug.
Owner:SIGNATURE THERAPEUTICS
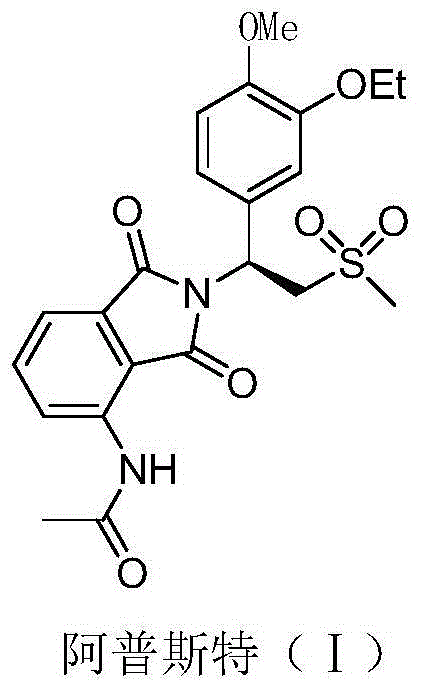
Preparation method for synthesizing apremilast intermediate
ActiveCN104447445AStable and cheapReaction is easy to controlOrganic chemistryOrganic compound preparationPhenyl groupSulfone
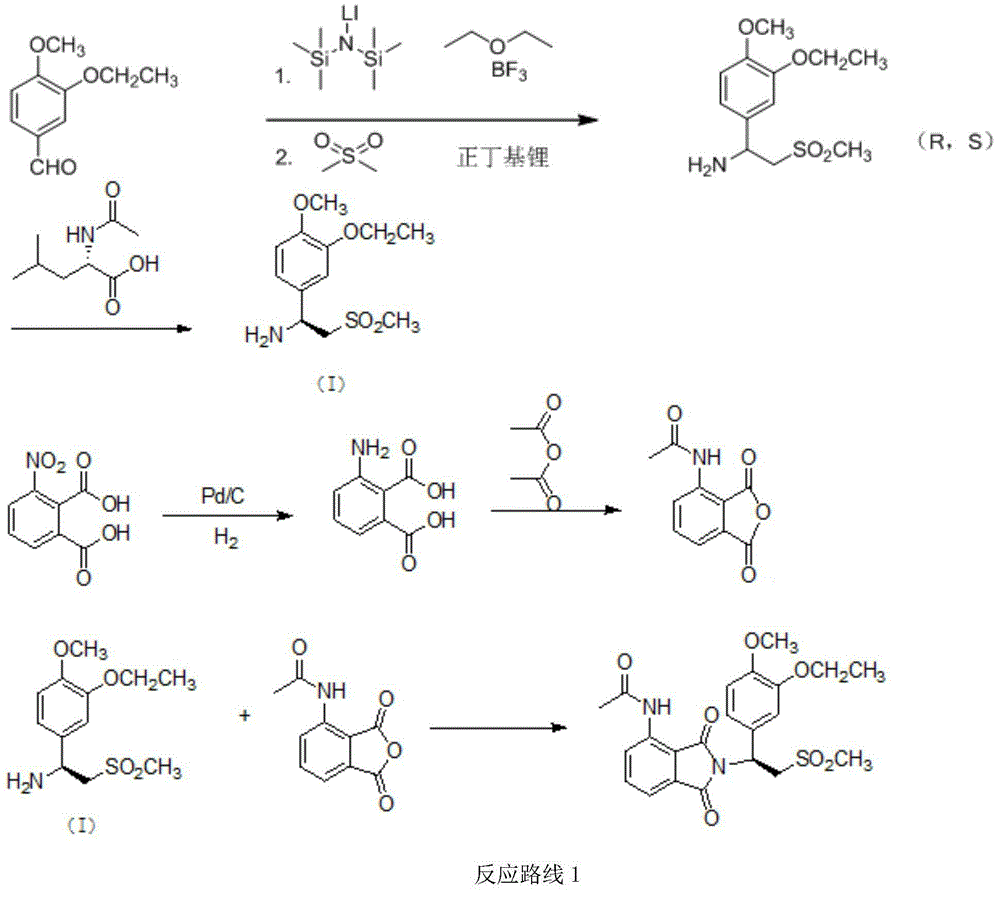
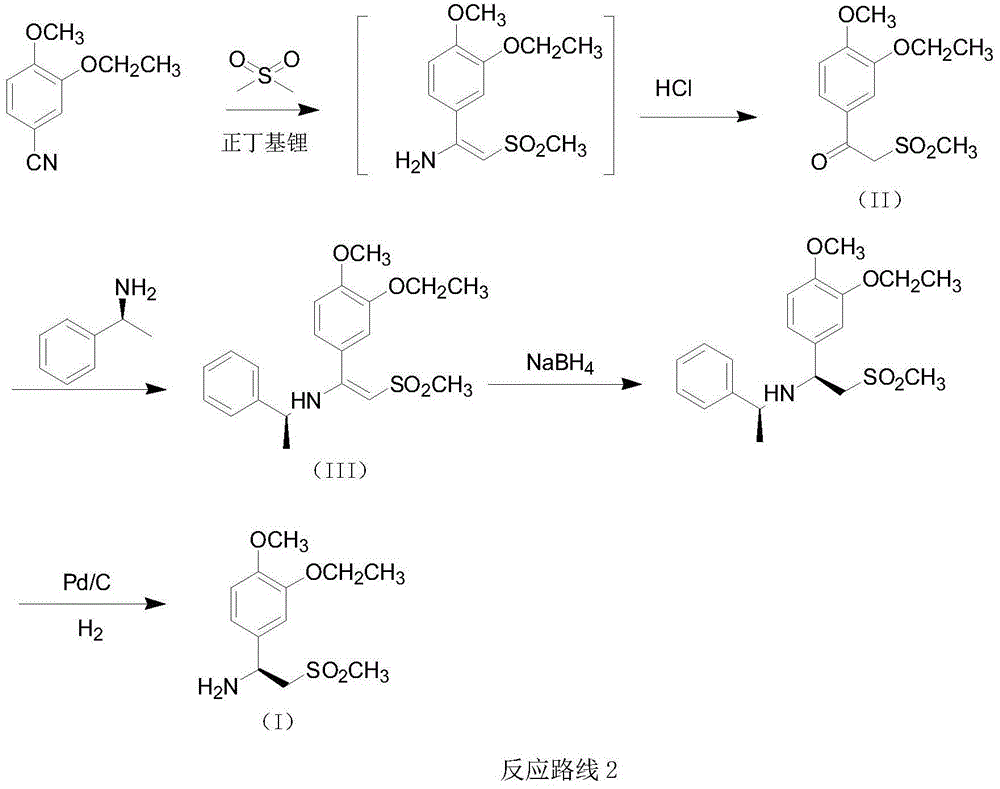
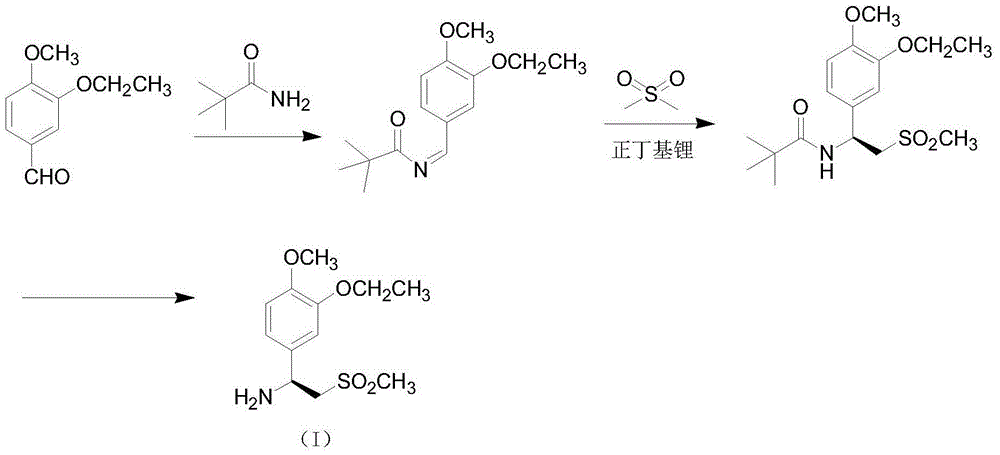
The invention relates to a preparation method for synthesizing an apremilast intermediate. The preparation method comprises the following steps of: carrying out condensation reaction on 3-ethoxyl-4-methoxyl-benzoate and dimethyl sulfone under an alkaline condition to generate 2-(3-ethoxy-4-methoxyphenyl)-1-methylsulfonyl acetone; reacting the compound II and chiral amine in the presence of an acidic catalyst to obtain 1-N-substituted amino-1-(3-ethoxyl-4-methoxyl) phenyl-2-methylsulfonyl ethylene (III), and directly hydrogenating the obtained compound III in the presence of a hydrogenation catalyst without separating the compound III to obtain a product (S)-1-(3-ethoxyl-4-methoxyl) phenyl-2-methanesulfonyl ethylamine (I), namely the apremilast intermediate, wherein the apremilast intermediate can be further prepared into N-acetyl L-leucinate. The invention also provides a preparation method of apremilast. The preparation method disclosed by the invention has the advantages of simple process flow, safety, environmental friendliness and low cost and is favorable to clean industrialized production.
Owner:XINFA PHARMA
Medicine-release system of compound Rifampicin
The present invention relates to a compound rifampin preparation, in particular, it relates to a medicine-releasing system of compound rifampin. Said system comprises rifampin and isoniazid, and further selectively can include pyrazinamide or pyrazinamide and etambol, which is characterized by that the isoniazid, pyrazinamide and etambol are gastric soluble, and the rifampin is enteric soluble orisoniazid is enteric soluble, and others are gastric soluble. Said preparation can be made into capsule, granules, tablet, multi-layer table and suspension preparation. Said invention also provides its preparation method.
Owner:SICHUAN LONG MARCH PHARMA CO LTD
Preparation method of salt formation of silodosin intermediate
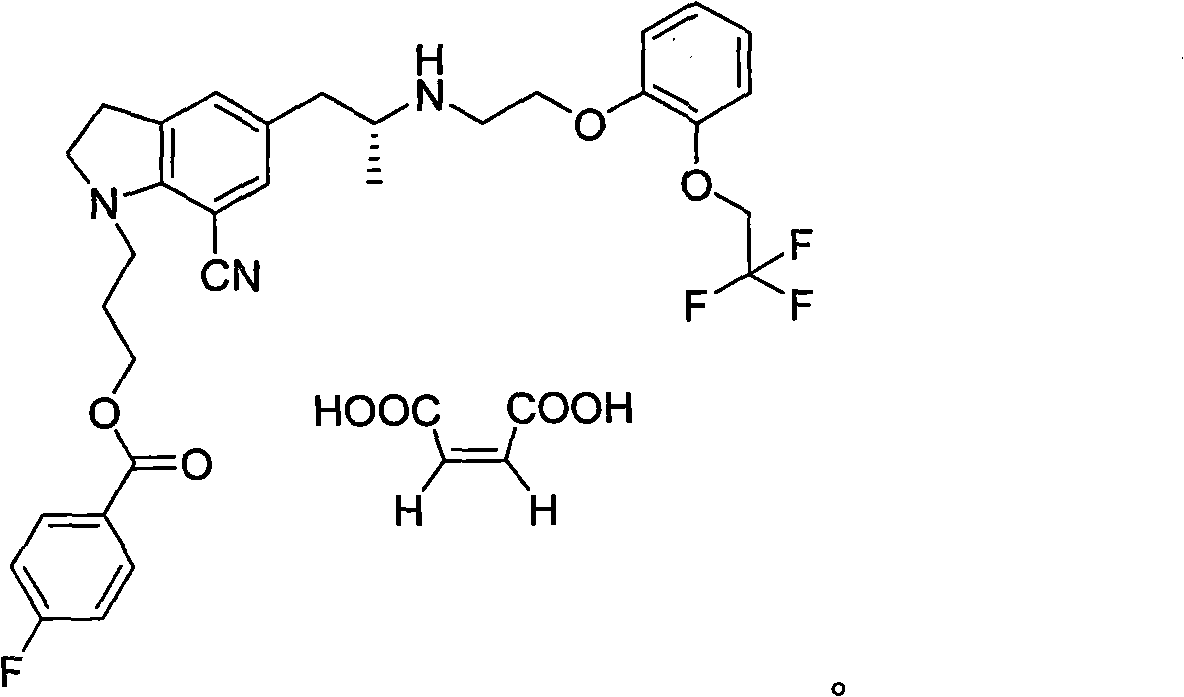
The invention relates to a method of maleate formation of a silodosin intermediate indoline compound 1 - (3 - (4 - fluoro benzoyl) hydroxypropyl) -5 - ((2R) -2 - (2 - (2 - (2,2,2 - trifluoroethoxyl) phenoxy) ethylamine) propyl) indoline -7 - cyano (compound (1)), i.e., a crude product of the compound (1) forms a salt with maleic acid in a mixed solvent of a good solvent and a poor solvent. The maleate of the compound can be stably obtained by the method, and the method has the advantages of good impurity removal effect, stable process, high yield, simpleness in operation and the like.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Research and control method of impurity B control method in clopidogrel
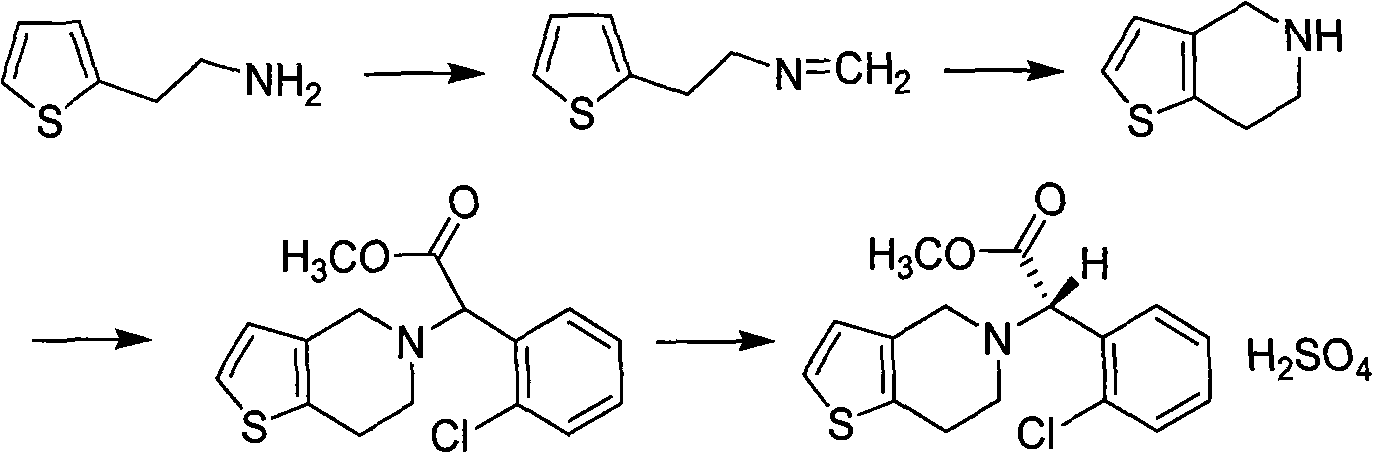
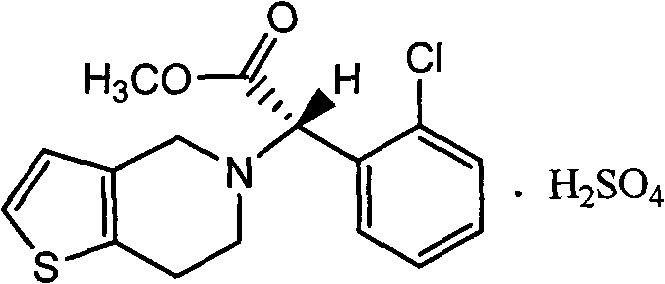
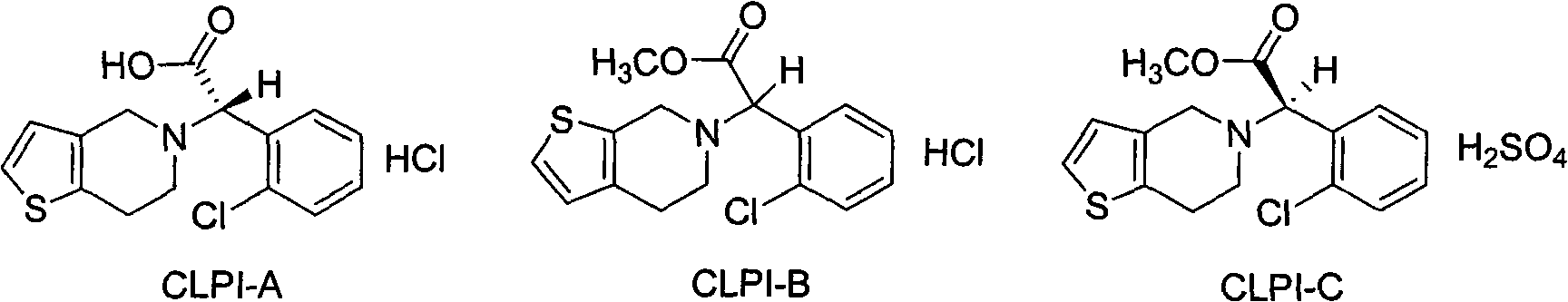
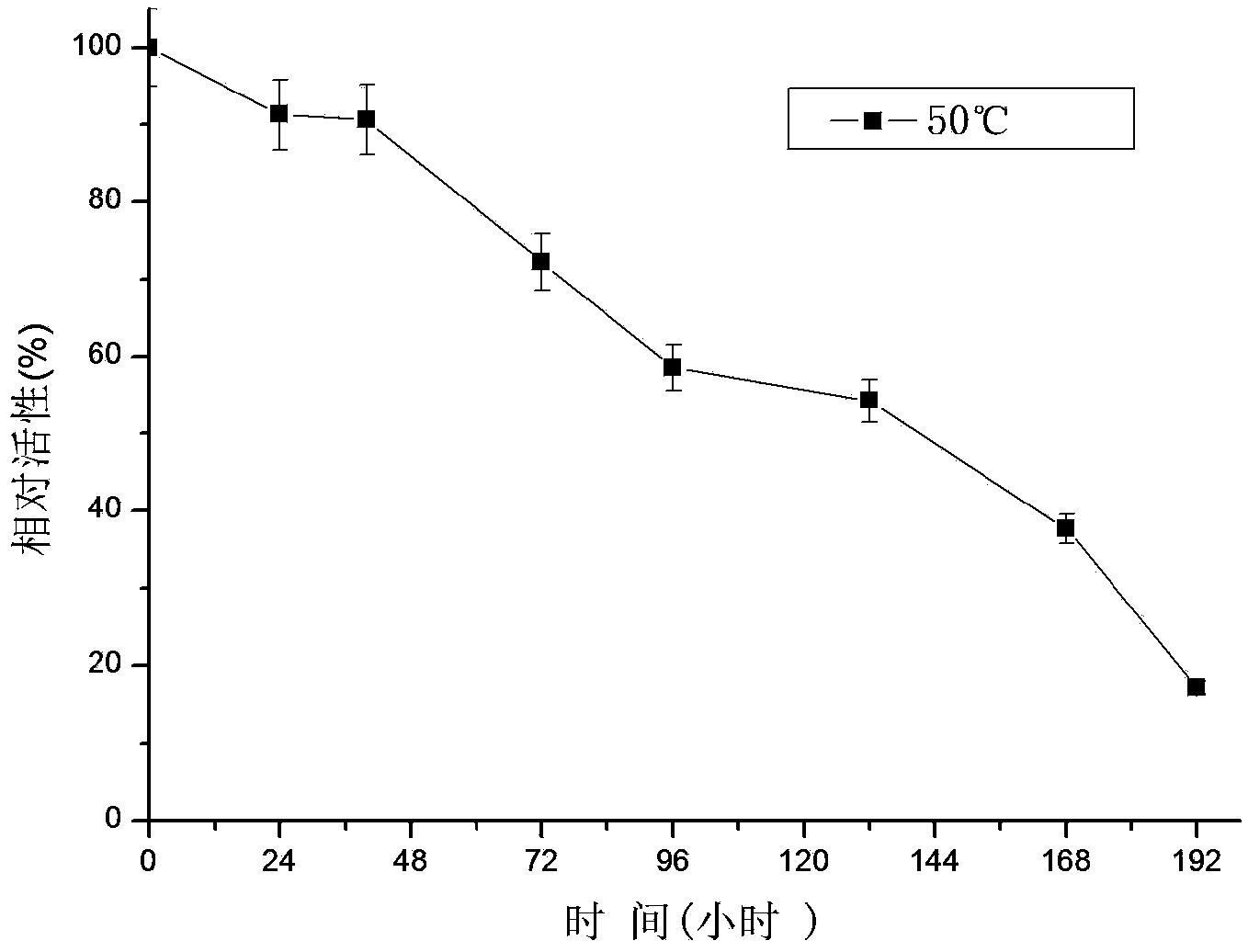
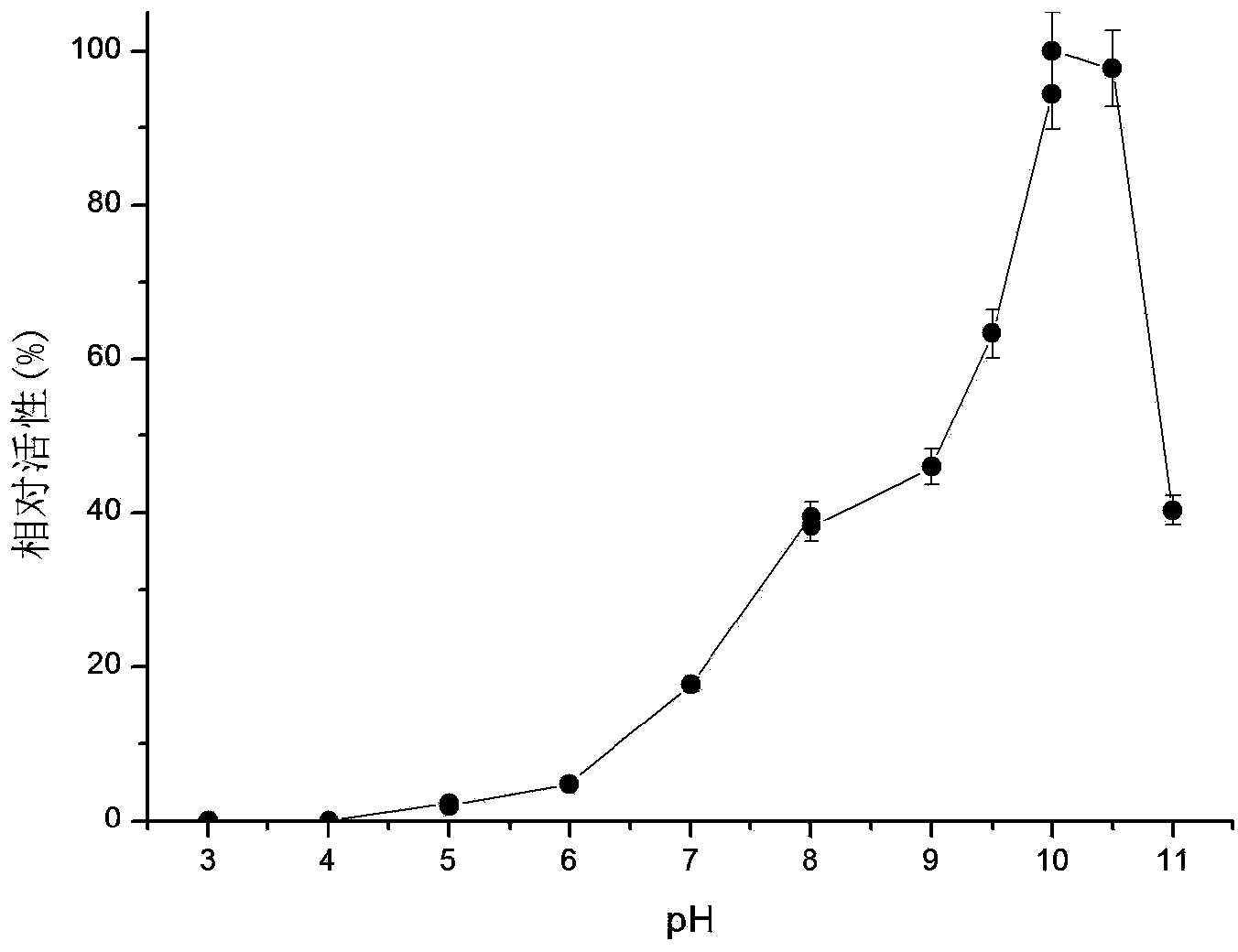
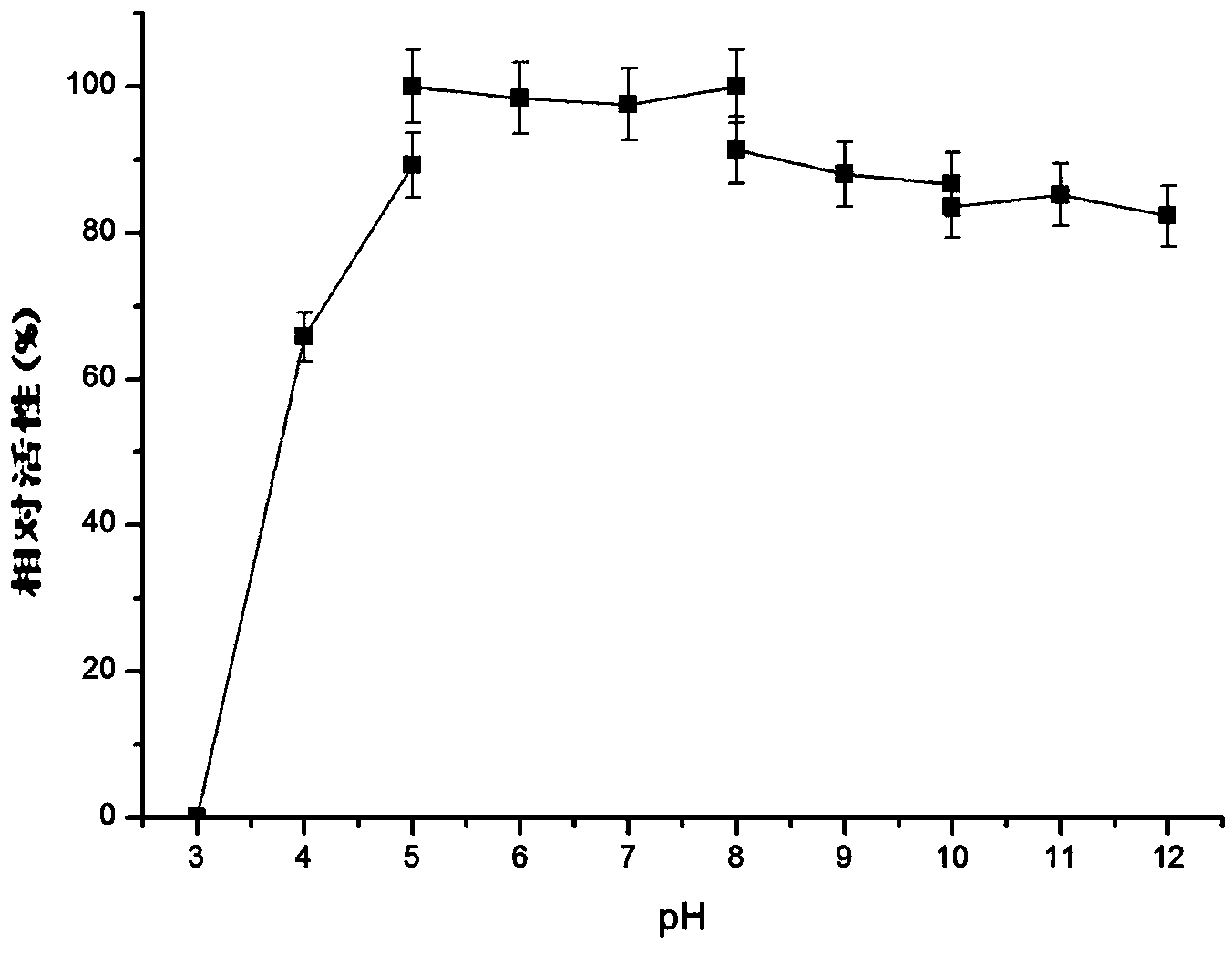
The invention relates to a research and control method of impurity B control method in clopidogrel, the method comprises the following steps: (1) preparing a batch or multiple batches of 2-thiophene ethylamine; (2) measuring the 3-thiophene ethylamine in (1) by a GC method; (3) selecting 2-thiophene ethylamine with 3-thiophene ethylamine content not more than 0.40% based on the step (2) as initial materials: (4) obtaining the clopidogrel with less impurity B, adopting the 2-thiophene ethylamine in the step (3) by the preparation of 2-thiophene ethyl methylene amine and the preparation of 4, 5, 6, 7-tetrahydrothieno[3, 2-c]pyridine.
Owner:CHINA RESOURCES SAIKE PHARMA
Method for synthesizing theanine
InactiveCN101597239ALow priceReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationL-theanineReaction speed
The invention relates to a method for synthesizing theanine, which comprises the following steps: aminating L-N-acyl glutamic acid-gamma-ester in 70-percent ethylamine aqueous solution to obtain L-N-acyltheanine; hydrolyzing the L-N-acyltheanine by adopting L-aminoacylase to remove the acyl group of the L-N-acyltheanine; and performing separation in ethanol aqueous solution to obtain high-quality L-theanine. The method combines the advantages of industrialized acyl hydrolyzation by the L-aminoacylase, uses cheap acyl group as a protective group, and uses the L-aminoacylase to hydrolyze the acyl group after reaction with the ethylamine aqueous solution to obtain the L-theanine. The method has the characteristics of low raw material price, low production cost, high selectivity, high reaction speed and high yield. The used raw materials and reagents are suitable for industrial production, the operation process is simple, and the product quality can be ensured.
Owner:TIANJIN PHARMA GROUP CORP
Method for synthesizing L-theanine through enzyme process
ActiveCN103409475AMake up for the shortcomings of poor stabilityHigh activityFermentationEscherichia coliChemical synthesis
The invention discloses a method for synthesizing L-theanine through an enzyme process, and belongs to the biotechnical field. The method is characterized in that a gamma-glutamyltranspeptidase gene is obtained through chemical synthesis, a gene engineering bacterium over-expressing gamma-glutamyltranspeptidase is constructed by treating Escherichia coli as a host bacterium, glutamine and ethylamine hydrochloride having different concentrations are acted by a recombinase, and theanine is efficiently produced at a temperature of 37-50DEG C under a pH value of 9.5-10.5. The enzyme source preparation process has the advantages of simplicity, low cost, and large enzyme amount, and the theanine production method has the advantages of simplicity, high conversion rate, high output, short time and the like, and is in favor of the industrialized amplification production.
Owner:JIANGNAN UNIV
Psycho-pharmaceuticals
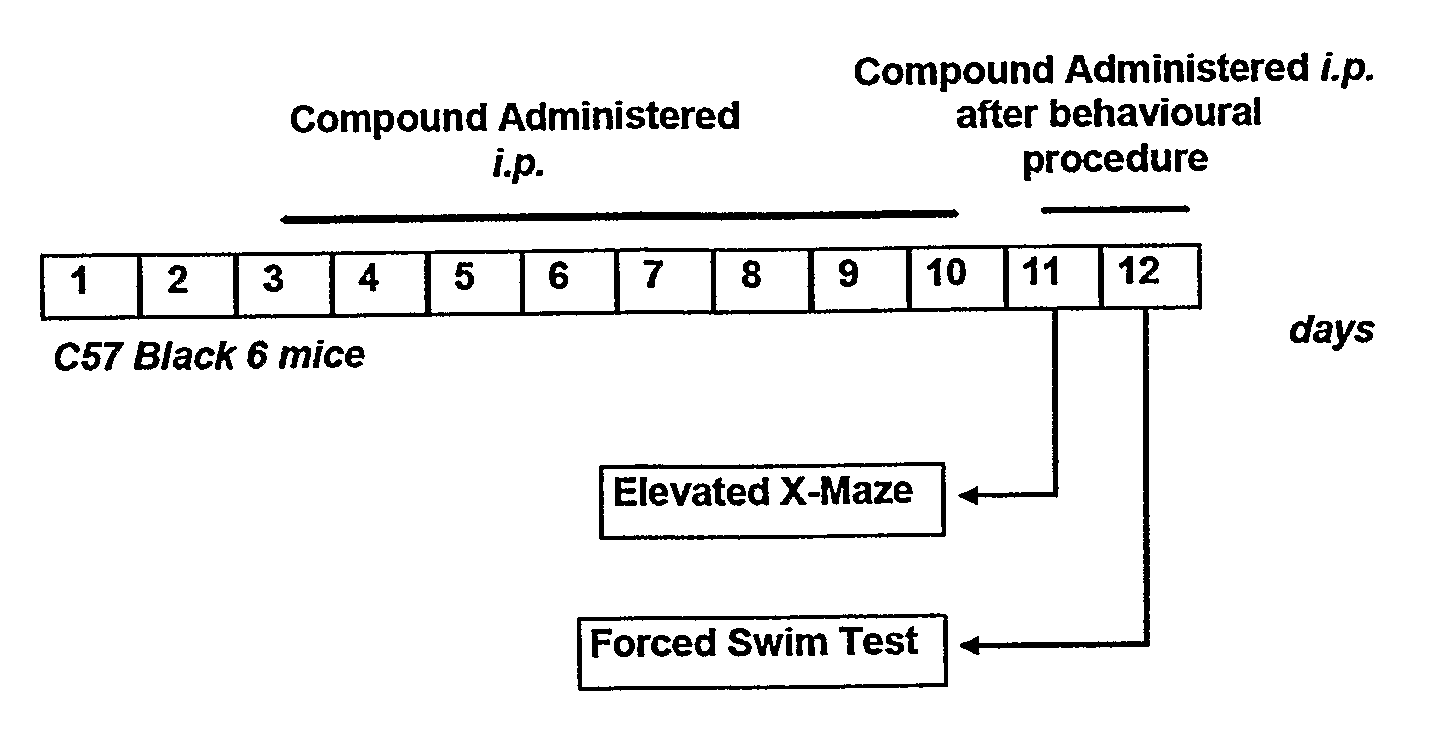
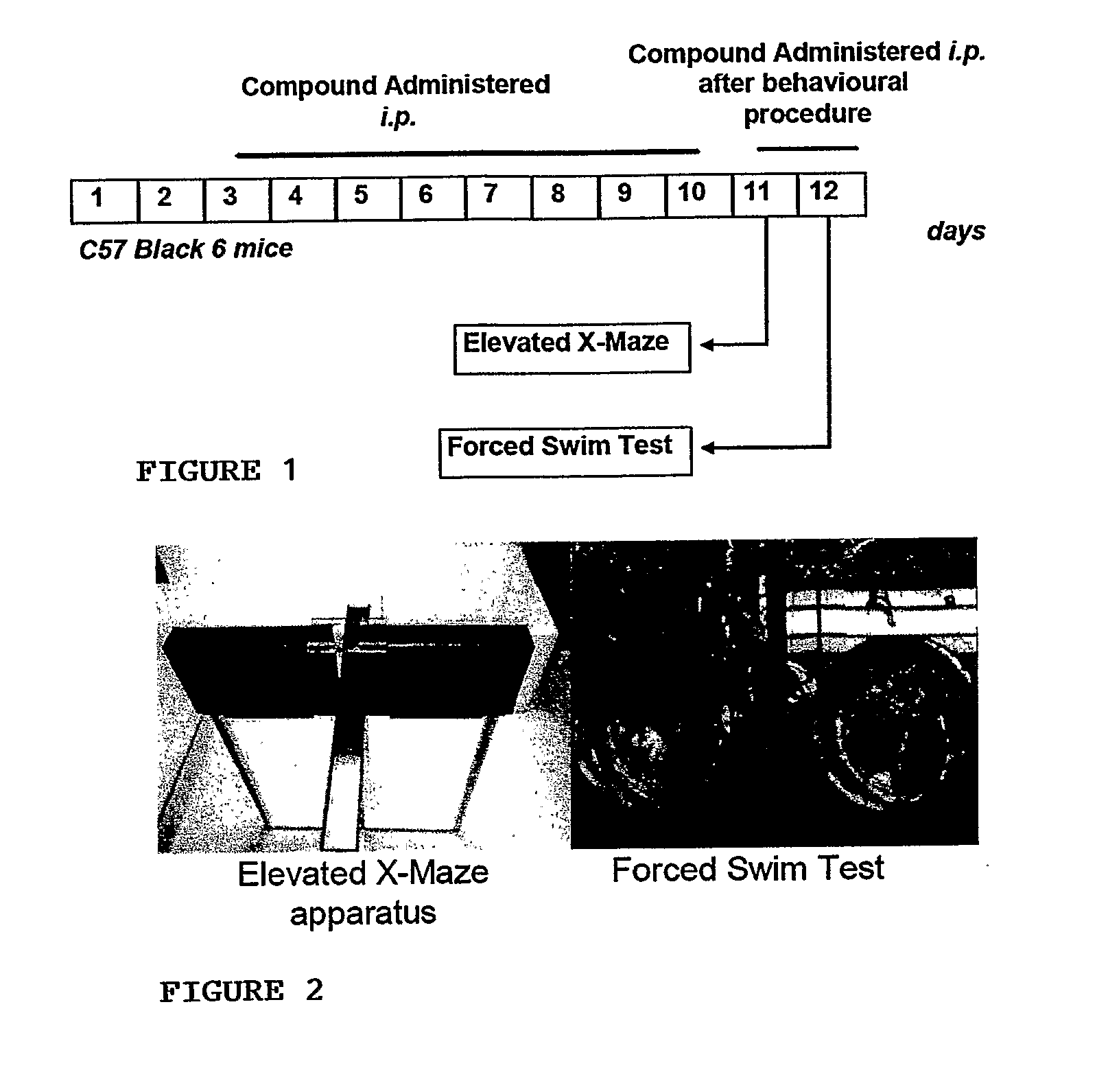
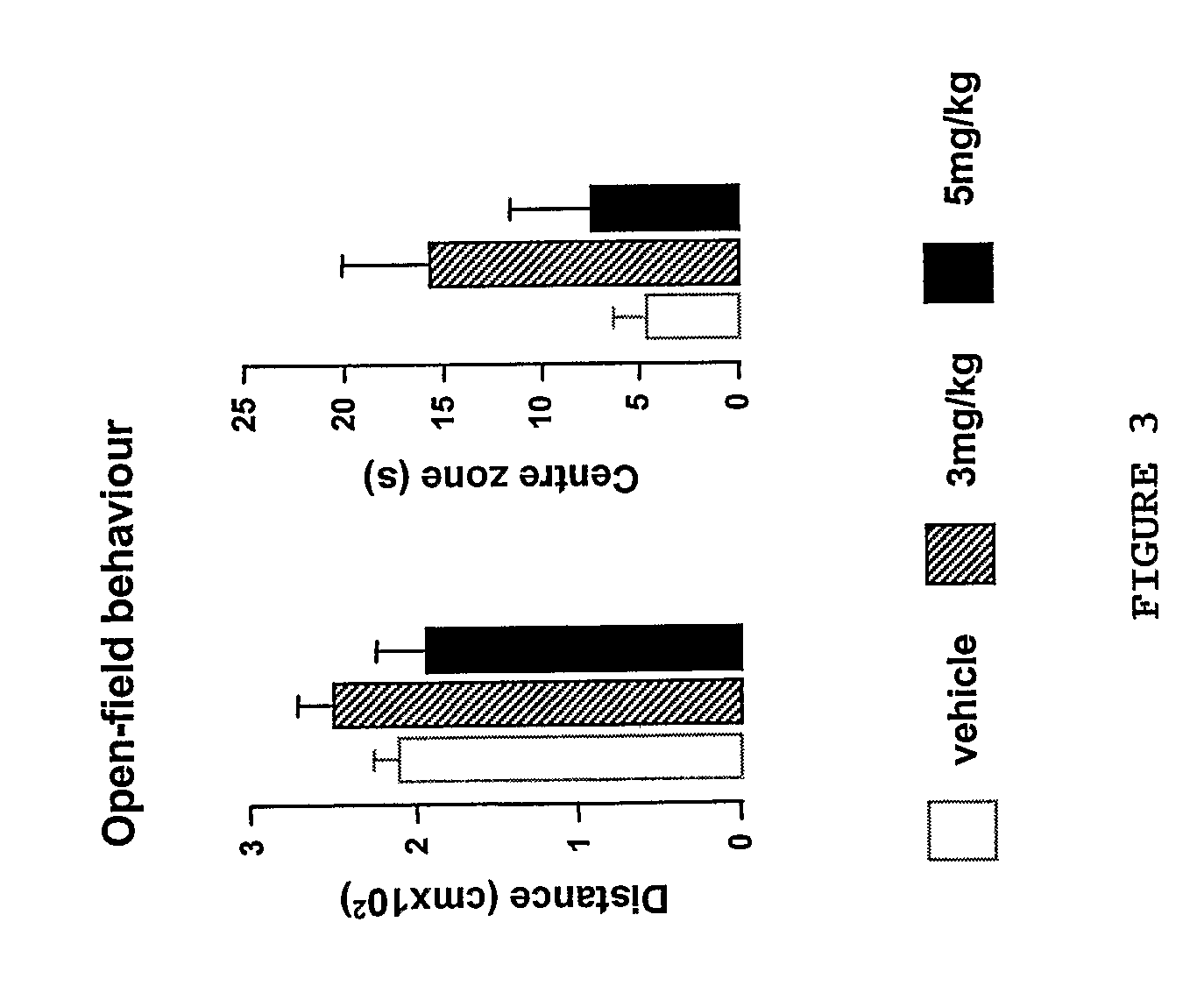
InactiveUS20110034562A1Improve cognitive abilityRelieve symptomsOrganic active ingredientsBiocidePsychoactive drugAgonist
The invention provides a selective Sigma 1 or Dopamine D3 receptor agonist or a 5HT2c receptor ligand for use in the treatment of symptoms of anxiety and / or depression associated with an affective disorder and / or symptoms associated with cognitive impairment disorder. Particularly useful are diphenhydramine derivatives of Formula (I) and particularly (2-[(4-butylsulfanylphenyl)-phenyl-methyl]sulfanyl-N,N-dimethyl-ethanamin) (Captodiamine).
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
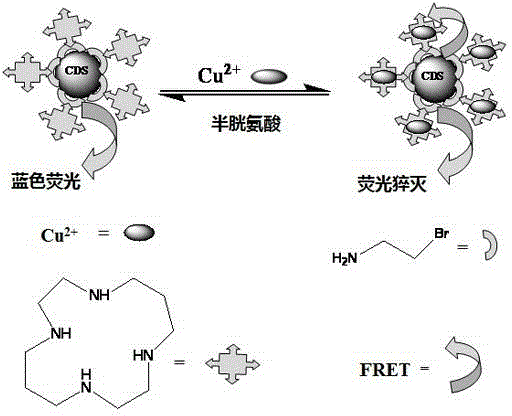
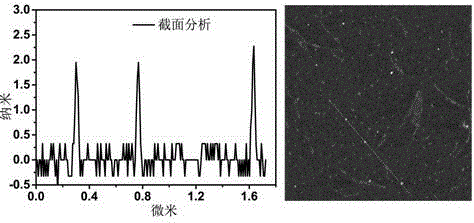
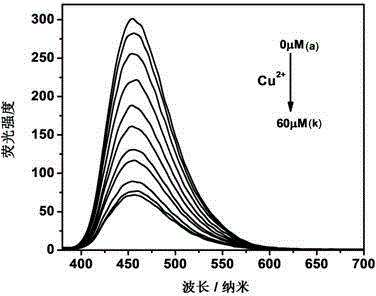
Carbon quantum dot sensor with copper ion and cysteine recognition functions, preparation method and application thereof
InactiveCN104357048AHighly selective detection and identificationThe synthetic route is simpleFluorescence/phosphorescenceLuminescent compositionsBromineIon
The invention discloses a carbon quantum dot sensor with copper ion and cysteine recognition functions, a preparation method and application thereof. The carbon quantum dot sensor is prepared by the following steps: taking citric acid and ethylene diamine as raw materials, adopting a microwave method to synthesize a carbon quantum dot containing carboxyl on the surface, then taking 2-bromoethylamine and 1,4,8,11-tetraazacyclotetradecane as surface functionalizing reagents, and utilizing a surface grafting technology. The carbon quantum dot sensor is good in dispersity in water, and can be used for double-selectivity fluorescence detection to the copper ion and cysteine. Compared with the existing detection technology, the carbon quantum dot sensor disclosed by the invention can detect trace of copper ion and cysteine in a pure water medium with high sensitivity and high selectivity, is simple in synthetic route, convenient to use, and suitable for large-scale synthesis and actual application in production, and has the enormous application prospect in the fields such as biology and environment detection.
Owner:HUNAN UNIV OF SCI & TECH
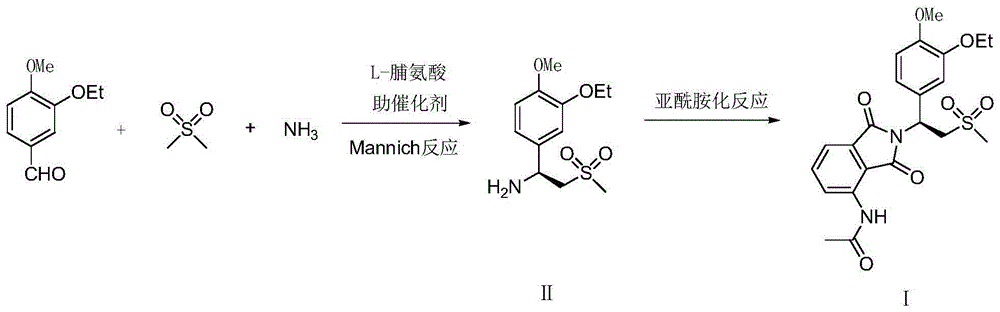
Preparation method for apremilast and intermediate of apremilast
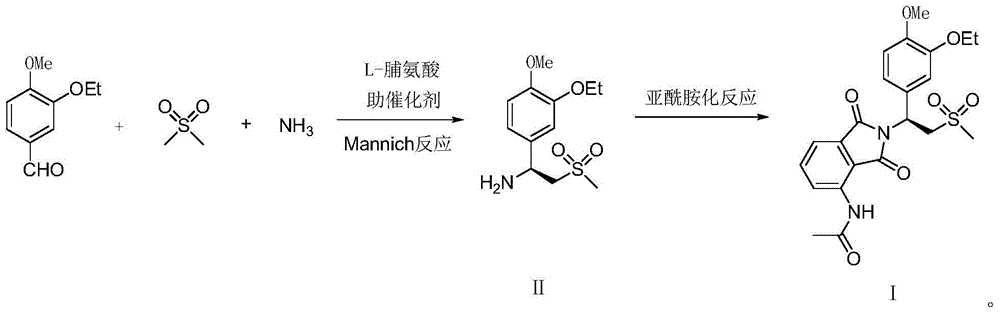
ActiveCN104447443AHigh purityRaw materials are easy to getOrganic chemistryOrganic compound preparationMannich reactionPhthalic anhydride
The invention relates to a preparation method for apremilast and an intermediate of the apremilast. The preparation method comprises the following steps: enabling 4-methoxy-3-ethoxybenzaldehyde to have Mannich reaction with methylsulfonylmethane and ammonia under the action of L-proline and a promoter to obtain an intermediate, (S)-1-(4-methoxy-3-ethyoxyl)phenyl-2-(methylsulfonyl)ethylamine (II), and then amidating the intermediate (II) and 3-acetamido-phthalic anhydride to prepare the apremilast (I). According to the preparation method for the apremilast and the intermediate of the apremilast, the raw materials are easily available, the flow path is short, the process is simple and convenient, the product has high optical purity, and the industrial production is safe and environmentally friendly.
Owner:XINFA PHARMA
Process for preparation of carvedilol
InactiveUS20060167077A1High technical contentReduce contentBiocideOrganic chemistryCarvedilolSolvent
The invention solves a new method of preparation of Carvedilol for pharmaceutical use. In the synthesis of Carvedilol a reaction of 4-(oxirane-2-ylmethoxy)-9H-arbazole (II) with 2-(2-methoxyphenoxy)ethylamine salts (IV) in the presence of a base, in an alcohol having the number of carbons C2 to C5 as a solvent, at an elevated temperature, is used. After processing of the crude reaction mixture crude Carvedilol is obtained, which is purified by crystallization from ethylacetate with an addition of activated carbon and the final substance is formulated by crystallization from ethylacetate.
Owner:SANECA PHARMA
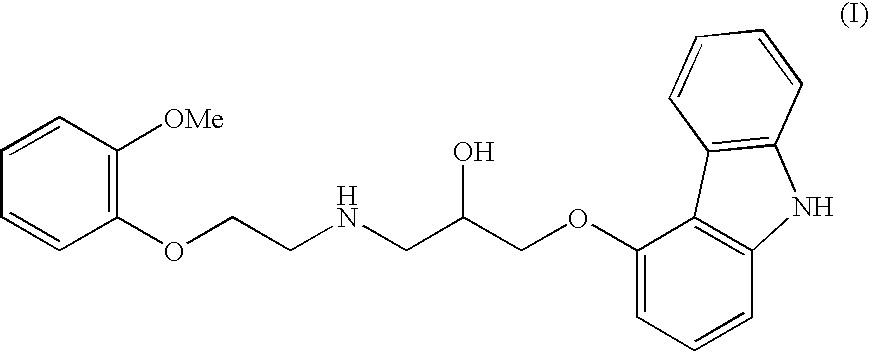
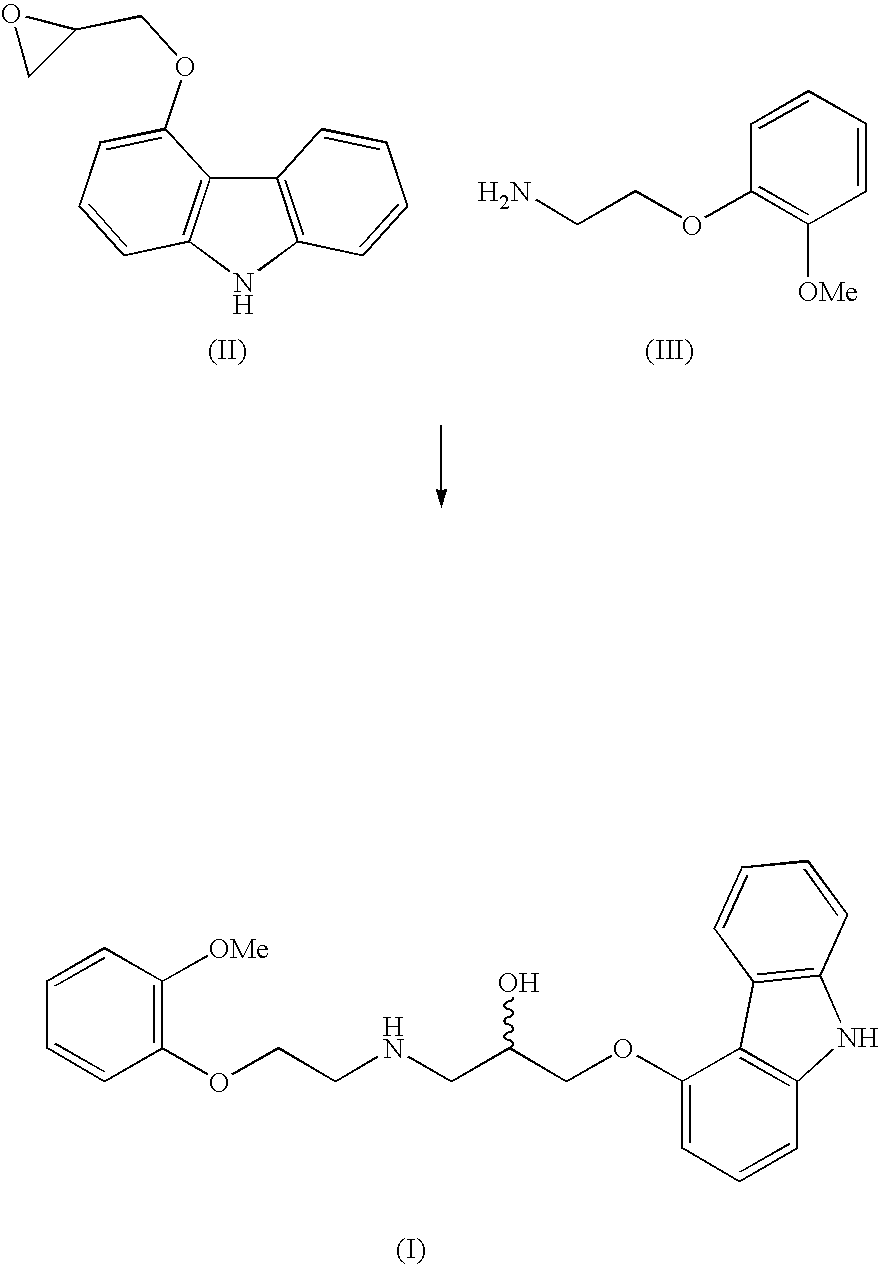
Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol
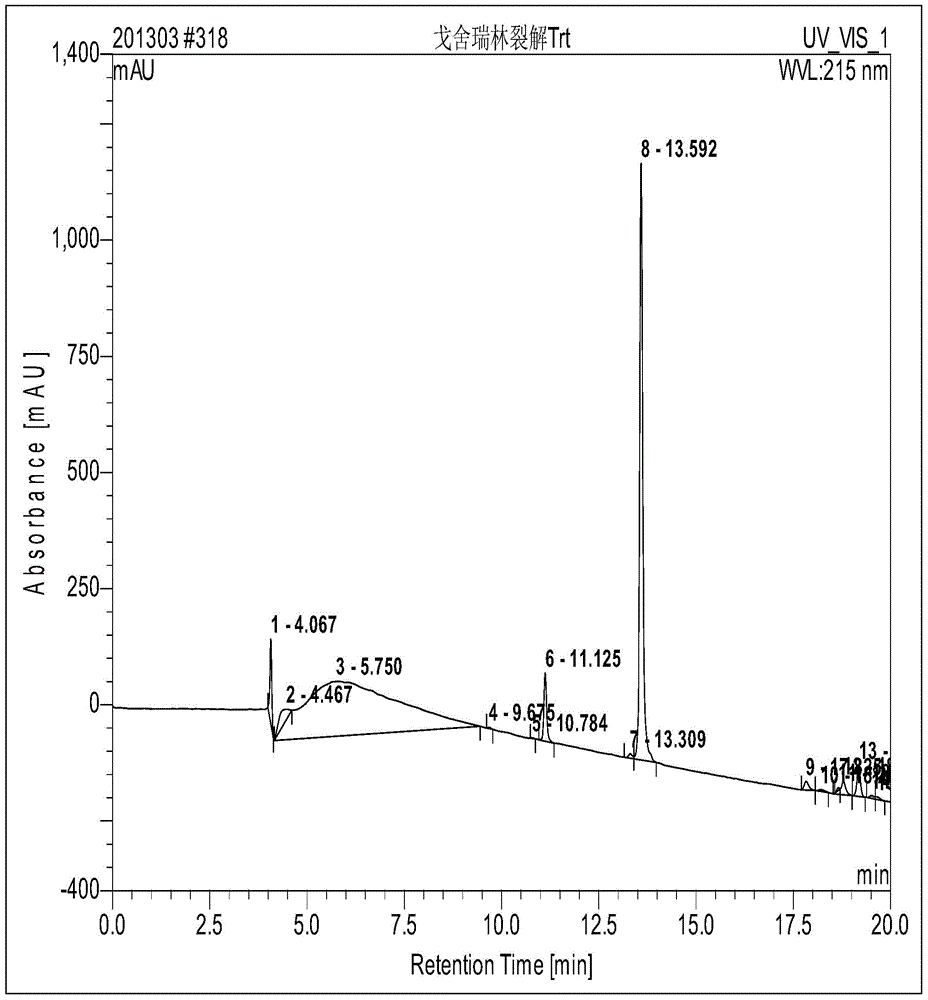
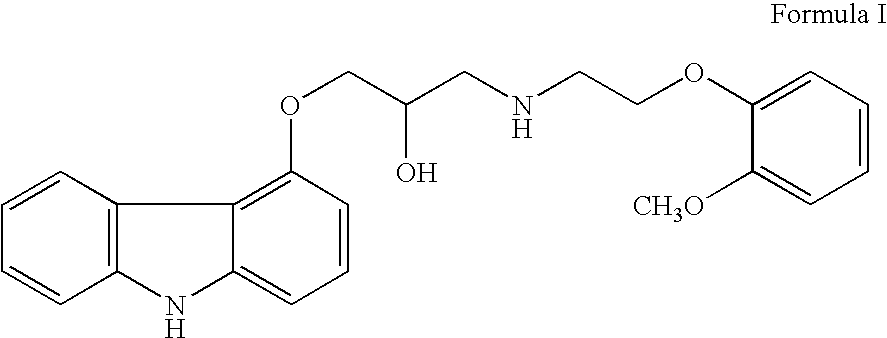
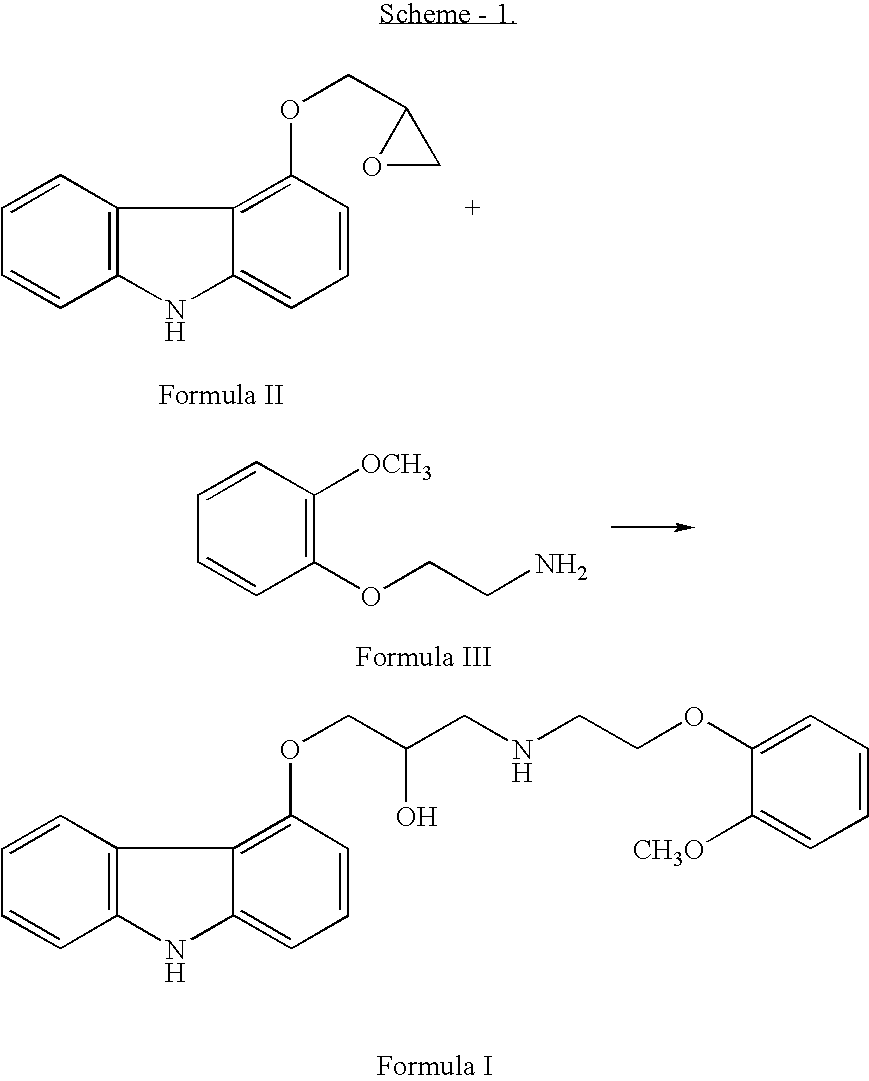
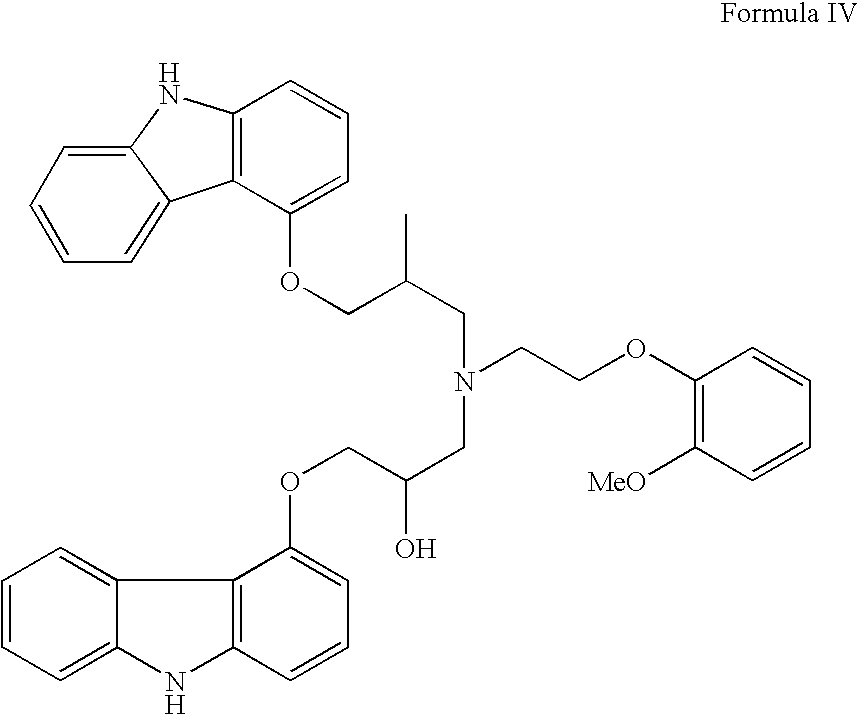
InactiveUS20070191456A1Few and simple stepReduce usageBiocideOrganic chemistryCarbazoleOrganic solvent
The present invention discloses a novel process for preparation of carvedilol by using eco friendly solvents to obtain the said carvedilol in high purity. The said process comprises, reacting 4-hydroxy carbazole of formula (IV) with epichlorhydrin in presence of an organic solvent and a base at temperatures between 10° C.-30° C.; further reacting the resultant 4-(2,3-epoxypropoxy)- carbazole of formula (II) with a salt of 2-(2-methoxyphenoxy)ethylamine of formula (III), preferably hydrochloride salt in presence of a base and a hydroxylic solvent at temperatures between 30° C.-90° C.
Owner:TARUR VENKATASUBRAMANIAN RADHAKRISHNAN +2
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylamine through ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Visible-light catalytic activity BiOBr-based heterojunction and preparation method thereof
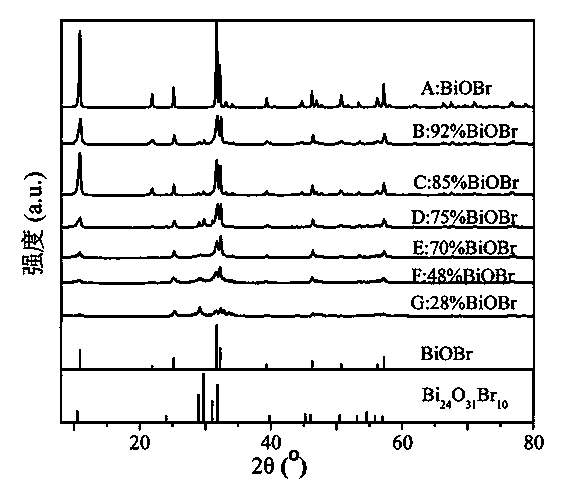
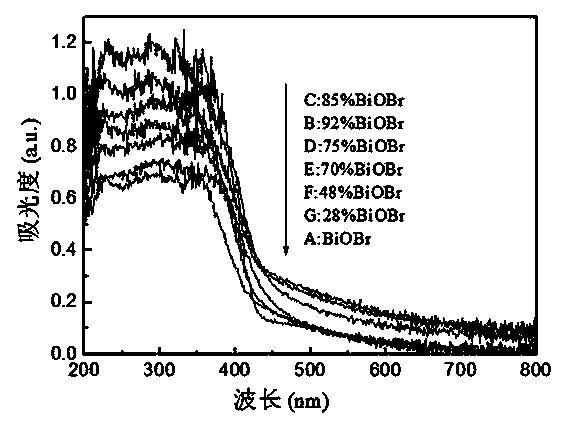
ActiveCN104190445AOvercomes the drawback of needing to add water to form a solutionEfficient separationPhysical/chemical process catalystsWater/sewage treatment by irradiationTetramethylammonium bromideHydrobromide
The invention discloses a visible-light catalytic activity BiOBr-based heterojunction material which is prepared from BiOBr and Bi24O31Br10 at a mass ratio of (28%-100%):(0%-72%). A preparation method of the BiOBr-based heterojunction material comprises the following steps: (1) mixing bismuth nitrate, urea, and one or more than one of tetramethyl ammonium bromide, tetraethyl ammonium bromide, 2-bromoethylamine hydrobromide and 3-bromopropylamine hydrobromide at a mass ratio of 1:(0.1-1.0):(0.1-1.0) to obtain a mixture, and heating the mixture until melting so as to obtain an ionic liquid; (2) heating the ionic liquid obtained in the step (1) until burning; and (3) collecting a solid generated after complete burning in the step (2), and cooling and grinding the solid to obtain the BiOBr-based heterojunction material. The preparation method is simple without requiring complicated equipment, and is short in time, high in yield, low in cost and suitable for industrial large-batched production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
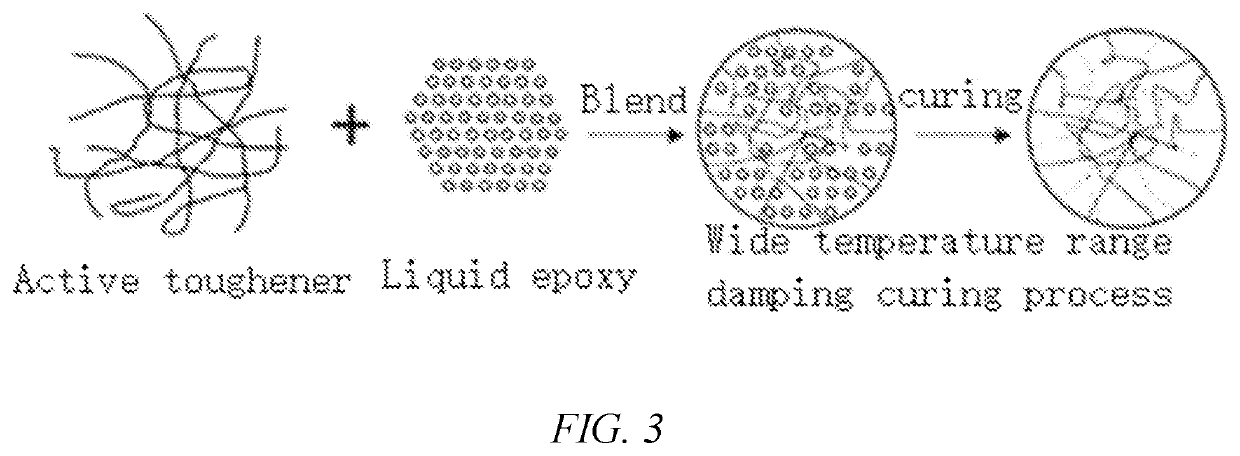
Anti-fatigue cold mixed epoxy resin material, preparation method and application thereof
ActiveUS20210253842A1Inhibitory activityReduced activityIn situ pavingsMacromolecular adhesive additivesEpoxyEndcapping
The invention relates to an anti-fatigue cold mixed epoxy resin material, preparation method and application thereof, comprising component A and component B with mass ratio of 1:1-10:1, component A comprising fluid epoxy resin, active toughener, active diluents, coupling agent and defoamer; component B is any one of or a mixture of two or more than two of alicyclic amine or amino terminated polyether, cyanoethylamine, phenolic modified amine or hydroxyalkyl modified amine. Introduced epoxy terminated organosilicon block polyurethane prepolymer breaks the limitation that elongation at fracture of epoxy resin system based on “sea-island structure” is difficult to break through 100%. The invention is suitable for bridge deck pavement of long-span cable bearing bridge, waterproof bonding material or used for airport pavement, municipal viaduct, ramp and other occasions with high requirements for fatigue resistance of pavement material.
Owner:SINOROAD TRANSPORTATION SCI & TECH CO LTD
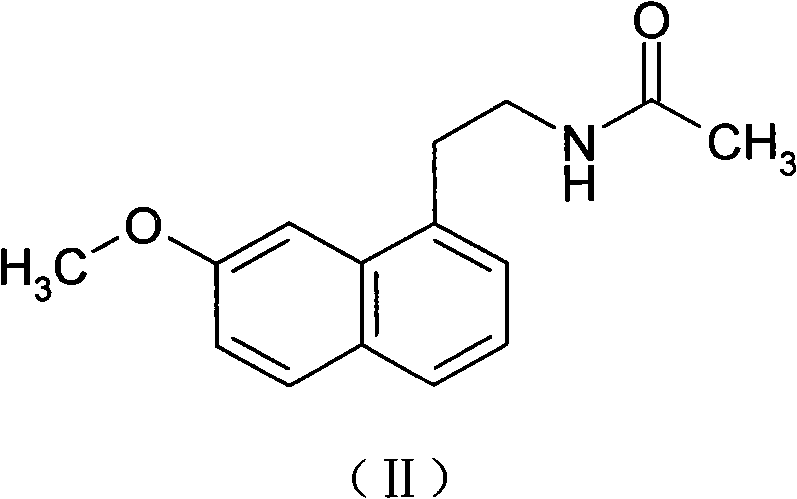
Preparation of agomelatine midbody, 2-(7-anisyl-1-naphthyl) ethylamine
ActiveCN101709036AReduce one step reactionHigh yieldOrganic compound preparationAmino-hyroxy compound preparationReaction stepAcetamide
The invention aims at providing a preparation method of 2-(7-anisyl-1- naphthyl) ethylamine (II) which is an important midbody of agomelatine. The preparation method uses 2- (7-anisyl-1-naphthyl) acetamide (VII) as an initiative raw material, only needs one reaction step, has higher yield, abolishes high-voltage hydrogenation, has mild conditions and does not need special devices.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
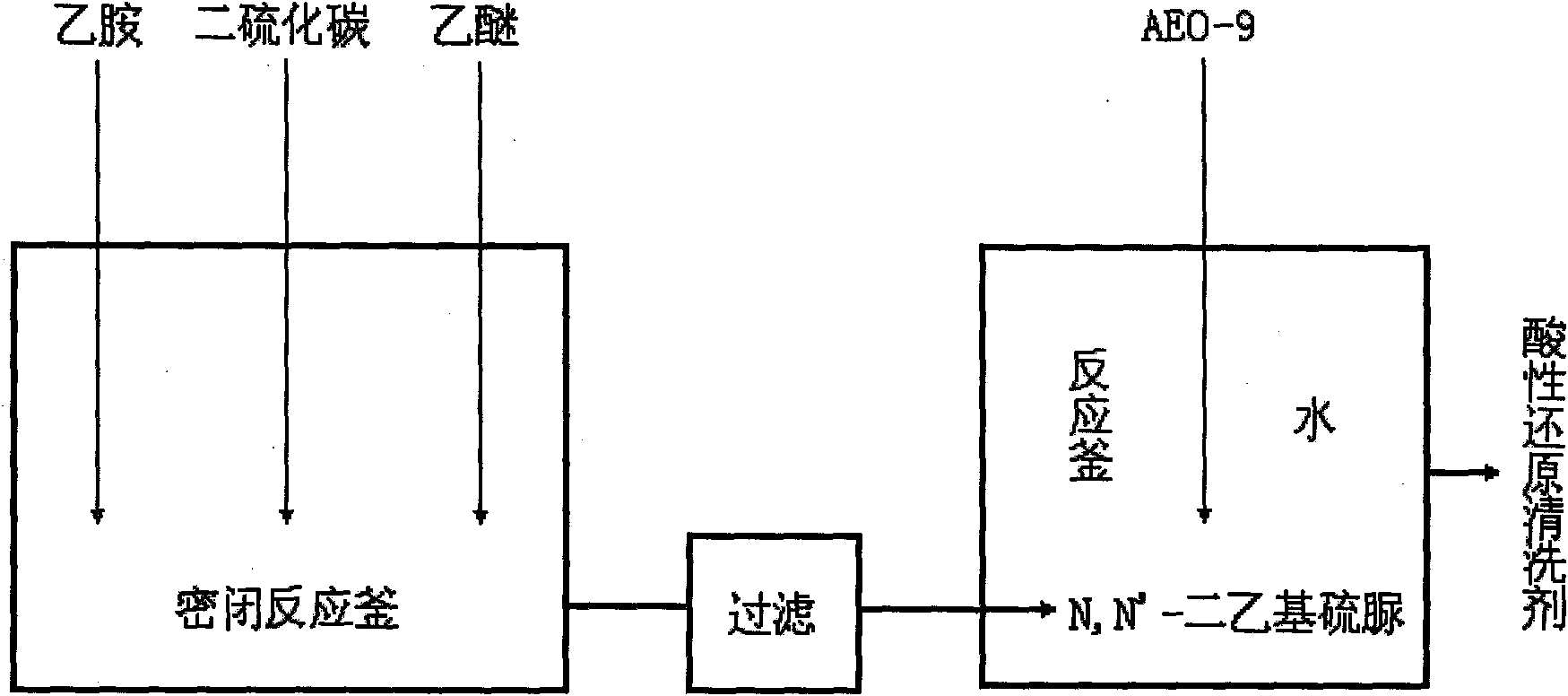
Acidic reduction cleaner
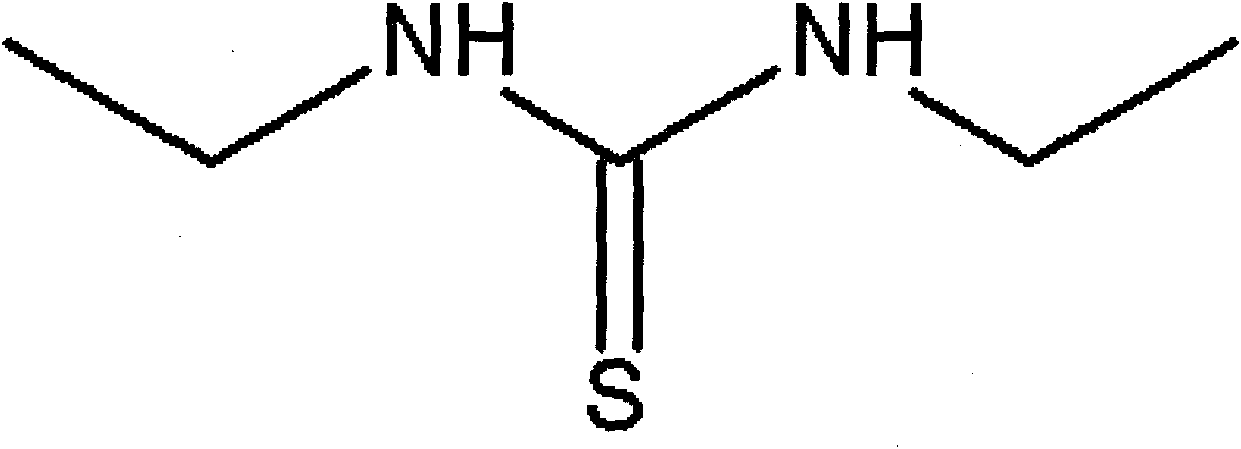
The invention relates to a printing and dyeing assistant for carrying out reduction cleaning on dyed polyester fabrics under acidic condition. Ethylamine reacts with carbon disulfide to generate N,N'-diethyl thiourea, and the N,N'-diethyl thiourea is compounded with fatty alcohol-polyoxyethylene ether to form an acidic reduction cleaner. In the process of reduction cleaning, the N,N'-diethyl thiourea is quickly in contact with the floating color of disperse dyes attached to fabric surfaces under the assistance of the fatty alcohol-polyoxyethylene ether to reduce carbonyl groups, ether bonds and other structures into hydrophilic hydroxyl groups, so that the floating color can be dissolved into water to be cleaned. Residual dyes in a dye bath and the cleaned floating color can also be reduced by the N,N'-diethyl thiourea for losing the capacity of secondary staining. On the premise of ensuring the cleaning effect, the invention can omit the steps of acid cleaning, water cleaning and the like, and the cleaning process can be directly carried out without discharging residual liquid when the dyeing rate of dyes is higher.
Owner:何鹰
Process for preparing glyphosate
ActiveCN1935816AReduce dosageIncrease dosageBiocideGroup 5/15 element organic compoundsDistillationSolvent
The invention discloses a new technology used to make glyphosate. It includes the following steps: weighting up each constituent according to the following mol ratio that methanol 3-10.5, tri-ethylamine 0.8-0.9, paraformaldehyde 1.95-2.05, glycine 1, dimethylphosphite 1.02-1.1, hydrochloric acid 2.9-3.34; de-polymerization that keeping the methanol, tri-ethylamine, and paraformaldehyde at 30-40 degree centigrade for 10-60min; adding glycine and keeping at 20-44 degree centigrade for 10-90min; condensation that adding dimethylphoshpite and keeping at 50-55 degree centigrade for 60-90min; acid hydrolysis that adding the composed liquid into hydrochloric acid and heating up to final 105-125 degree centigrade; crystallizing that producing glyphosate hydrochloride, then decompression, distillation, removing solvent methanol and excess hydrochloric acid. The produced glyphosate has the advantages of high yield, good quality, and low energy consumption.
Owner:SICHUAN LESHAN FUHUA TONGDA AGRO-CHEM TECH CO LTD
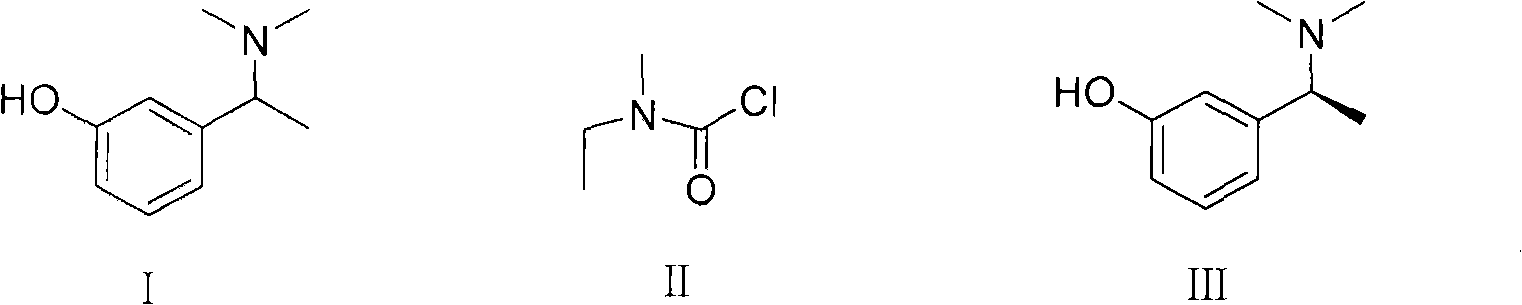
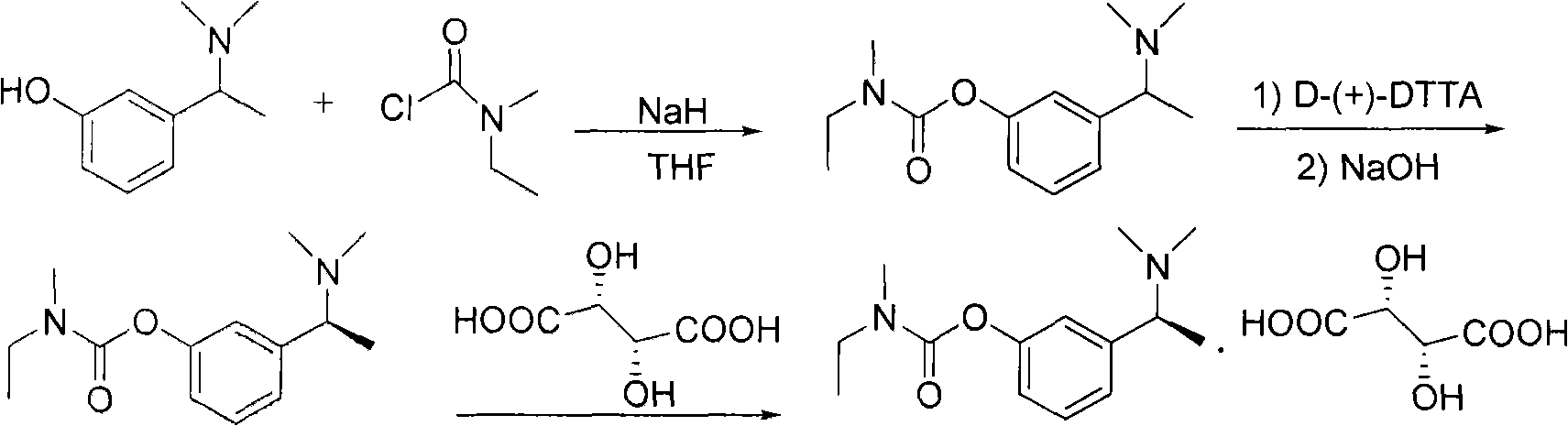
Method for preparing rivastigmine hydrogen tartrate and application thereof
InactiveCN101580482AEasy to operateLow costNervous disorderCarbamic acid derivatives preparationPhosgeneTriphosgene
The invention relates to a method for preparing rivastigmine hydrogen and tartrate thereof, which comprises the following steps: taking metamethoxyacetophenone as an initial raw material, and obtaining 1-(3-methoxyphenyl)ethanol by the reduction; then performing the chlorination to obtain 1-(chloroethyl)-3-methoxyphenyl; then reacting the1-(chloroethyl)-3-methoxyphenyl with dimethylamine hydrochloride to obtain 1-(3-methoxyphenyl)-N, N-dimethylethanamine; demethylating the reaction product to obtain 3-[1-(dimethylamino)ethyl]phenol; then performing salt formation resolution with (s)-(+)-camphor-10-sulfonic acid, recrystallizing, and dissociating to obtain (s)-3-[1-(dimethylamino)ethyl]phenol; then taking ethylamine as a raw material to react with ethyl formate to obtain formylethylamine; then reacting the formylethylamine with phosphorus oxychloride to obtain an imine intermediate; reducing the imine intermediate by sodium borohydride to obtain ethyl methyl amine; then reacting the ethyl methyl amine with triphosgene to obtain N-methyl-N-ethylformyl chloride; and finally using (s)-3-[1-dimethylamino)ethyl]phenol to condensate with the N-methyl-N-ethylformyl chloride, and then performing salt formation with levotartaric acid to obtain the rivastigmine hydrogen tartrate. The method has the advantages of easily-obtained raw materials, simple and convenient operation, low cost, high yield and small pollution, and is a brandnew synthesis route at present.
Owner:SHENYANG PHARMA UNIVERSITY
Disinfectant health-care aromatic hand paper towel and preparation method thereof
ActiveCN104846685ALow costReduce manufacturing costCosmetic preparationsToilet preparationsAntibacterial agentAntibacterial property
The invention discloses a disinfectant health-care aromatic hand paper towel and a preparation method thereof. The hand paper towel comprises the following components: softwood sulfite pulp, bamboo-pulp paper fiber, straw pulp, a cationic starch solution, a wet strength agent, a powerful antibacterial agent, an aromatic agent and titanium dioxide, wherein the wet strength agent is polyethylene ethylamine resin, the powerful antibacterial agent is composed of pure-oxygen-based quaternary ammonium salt, Sophora flavescens, common cnidium fruit, honeysuckle, borneol, isatis root, ethanol and distilled water, and the aromatic agent is composed of chitosan, ethanol, essence extract, deionized water, glycerin and triphosphoric acid. The prepared disinfectant health-care aromatic hand paper towel in the invention has the characteristics of no toxicity, harmlessness, environmental protection, highly-efficient antibacterial property and emission of aroma, can efficiently avoid contact-type cross infection with bacteria in public places and is safer and more secure to use.
Owner:ZHEJIANG HUACHUAN IND GRP
N-alkyl-2-phenoxyethanamines, their preparation and use
The present invention provides a compound having the structure: (structurally represented) wherein R1, R2, R3, R4, and R5 are each independently H, halogen, CF3 or C1-C4 alkyl; R6 is alkyl; A is absent or present, and when present is —C(O)— or —C(O)NH—; B is substituted or unsubstituted monocycle, bicycle, heteromonocycle, heterobicycle, benzyl, CO2H or (C1-C4 alkyl)-CO2H, wherein when B is CO2H, then A is present and is —C(O)—, or a pharmaceutically acceptable salt thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Graphene-butadiene-acrylonitrile latex compound slurry and preparation method and application thereof
InactiveCN107043477AThe preparation process is controllableImprove stabilityGlovesDomestic articlesLatex gloveAcrylonitrile
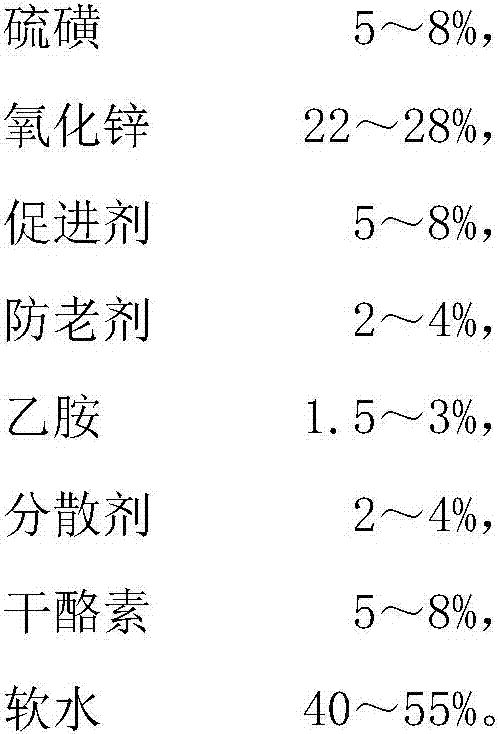
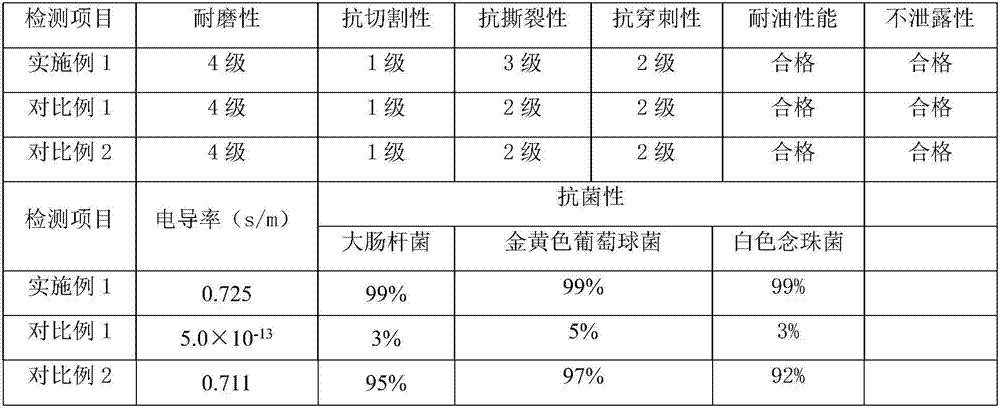
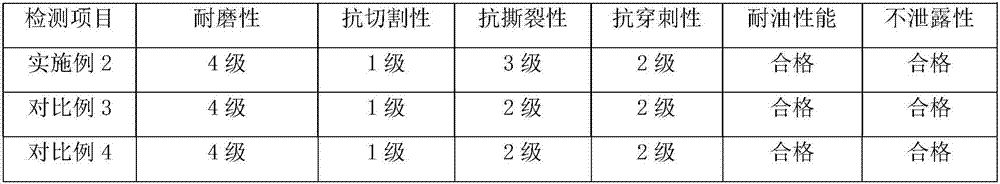
The invention discloses graphene-butadiene-acrylonitrile latex compound slurry and a preparation method and application thereof. The compound slurry comprises the following components in parts by weight: 100 parts of butadiene-acrylonitrile rubber and 0.025-5 parts of graphene. The compound slurry also comprises 4-6 parts of a ball-milled sulfur material. Based on the total weight of the ball-milled sulfur material, the ball-milled sulfur material comprises the following components in percentages by weight: 5-8% of sulfur, 22-28% of zinc oxide, 5-8% of an accelerant, 2-4% of an anti-ageing agent, 1.5-3% of ethylamine, 2-4% of a dispersant, 5-8% of casein and 40-55% of soft water. A pair of graphene-butadiene-acrylonitrile latex gloves prepared from the composite slurry is flexible in texture, comfortable to wear, high in tear strength, antibacterial, antistatic, small in stress at definite elongation and good in anti-ageing property, and fully exerts the advantages of graphene which is high in conductivity, high in strength, resistant to friction, high in bacteriostatic property, and the like.
Owner:NANTONG QIANGSHENG SAFETY PROTECTION TECHNOLOGY CO LTD
Indoline derivative as well as preparation method and application thereof
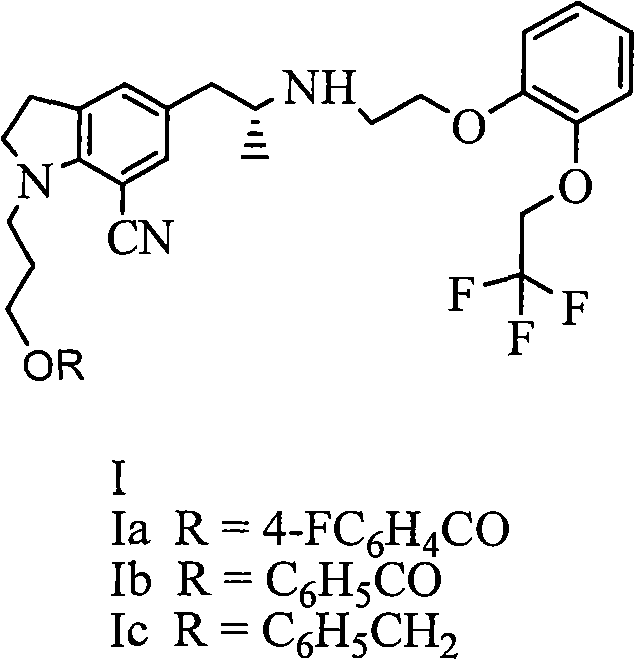
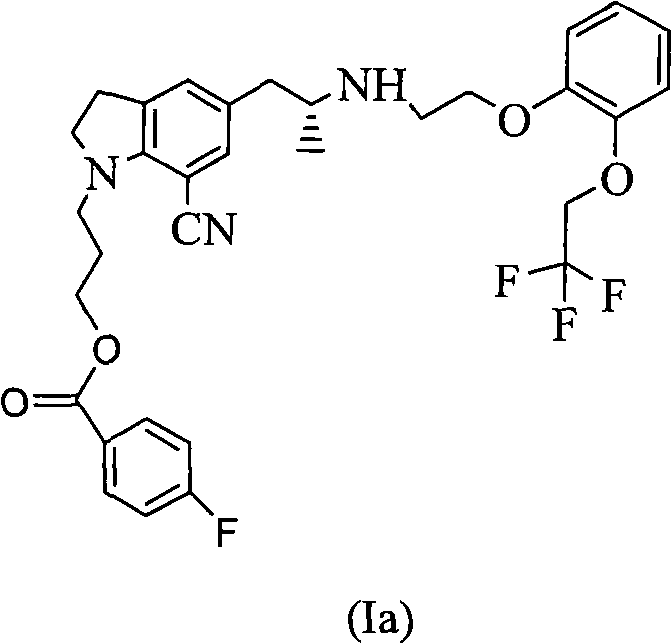
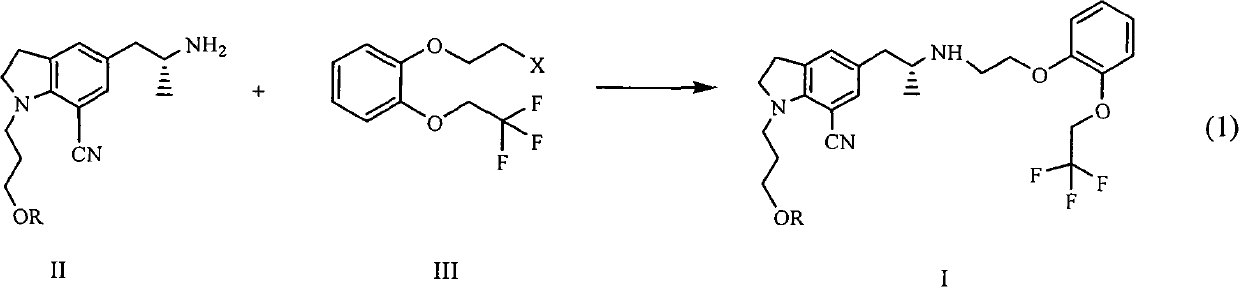
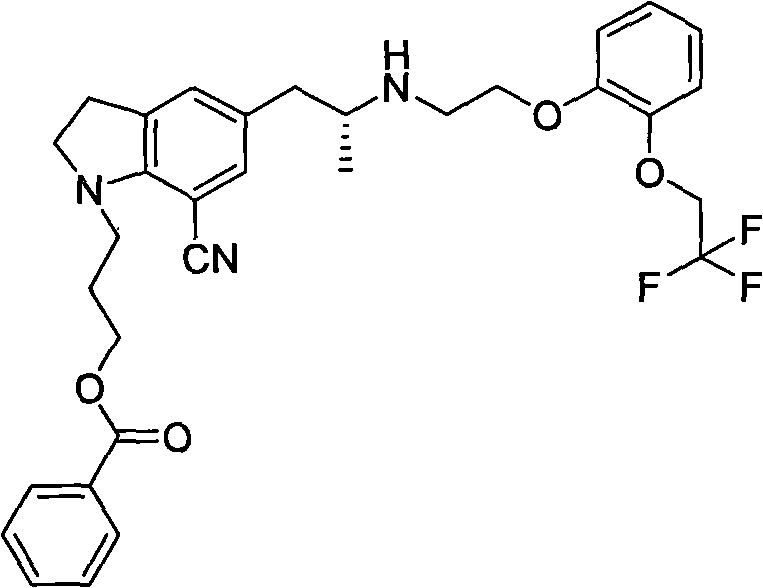
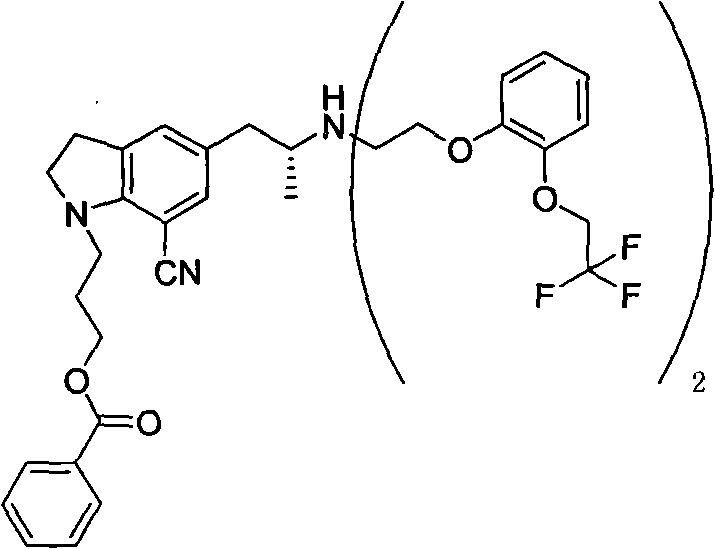
The invention provides a preparation method of 1-(3-protecting propyl)-5-((2R)-2-(2-(2-(2,2,2-trifluoroethyoxyl)phenoxy)ethylamine)propyl)indoline-7-cyan and 1-(3-(4-flurobenzoyl)hydroxyl propyl)-5-((2R)-2-(2-(2-(2,2,2-trifluoroethyoxyl)phenoxy)ethylamine)propyl)indoline-7-cyan shown as in a specific compound structure (Ia) in the specification. The method is characterized in that water is used as a solvent and the yield is high. The 1-(3-(4-flurobenzoyl)hydroxyl propyl)-5-((2R)-2-(2-(2-(2,2,2-trifluoroethyoxyl)phenoxy)ethylamine)propyl)indoline-7-cyan shown as in the specific compound structure (Ia) can be used as an intermediate to be applied to the preparation of an indoline derivative. The method of the invention is simple in operation and high in yield.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +2
Goserelin acetate solid-phase synthesis method
ActiveCN104910257ATo solve such a drawback that cannot be monitoredLow costLuteinising hormone-releasing hormonePeptide preparation methodsSide chainEthylic acid
The invention relates to a goserelin acetate solid-phase synthesis method, which comprises the following steps: HBTU / DIPEA is employed as a condensation system, Fmoc-Ser-OH, Fmoc-Trp-OH are successively coupled; a whole-protection lysate with corresponding voluminal amount according to 10 times of resin weight is added, a carrier 2-CTC Resin in an intermediate is removed, all side chain protective groups are reserved; the whole-protection lysate is adjusted to slight alkaline by using DIPEA(N,N-diisopropylethylaine), semicarbazide hydrochloride and PyBop(1H-benzotriazole-1-oxygen tripyrrole alkyl hexafluorophosphate) (used for a coupling agent of peptide) are added in the whole-protection lysate for reaction coupling, a goserelin peptide solution with the side chain protective group is obtained; the lysate with 20% of TFA / DCM is added in a freezing ether for settling to obtain the white solid crude peptide; the white solid crude peptide is dried under vacuum for solving by methyl alcohol, ammonium formate and Pa / c are added for a hydrogenation reaction to remove the side chain protective group in a peptide sequence. According to the invention, side reaction phenomena can be avoided, target peptide purity is increased, yield is high, operation is convenient and feasible, the intermediate can be tracked and controlled, and the whole process is benefit for enlarged production.
Owner:苏州天马医药集团天吉生物制药有限公司
Process for the preparation of carvedilol and its salts
Disclosed herein is a process for preparation of carvedilol substantially free from its bis-impurity comprises the reaction of 4-(2,3-epoxypropoxy)carbazole and 2-(2-methoxyphenoxy)ethylamine in a polar aprotic solvent media; followed by isolation of carvediol from the reaction mass as an acid addition salt and subsequent conversion into pure carvedilol.
Owner:IPCA LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol](https://images-eureka.patsnap.com/patent_img/410334a5-f68c-43f7-9f60-468d1a1f7aab/US20070191456A1-20070816-C00001.png)
![Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol](https://images-eureka.patsnap.com/patent_img/410334a5-f68c-43f7-9f60-468d1a1f7aab/US20070191456A1-20070816-C00002.png)
![Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol Novel process for the preparation of 1-(9h-carbazol-4-yloxy)-3-[[2-(-methoxyphenoxy)-ethyl] amino]-propan-2-ol](https://images-eureka.patsnap.com/patent_img/410334a5-f68c-43f7-9f60-468d1a1f7aab/US20070191456A1-20070816-C00003.png)