Preparation of agomelatine midbody, 2-(7-anisyl-1-naphthyl) ethylamine
A methoxyl and naphthyl technology, applied in the field of medicine, can solve the problems of high equipment requirements, low total yield, difficult industrial scale, etc., and achieve the effect of reducing one-step reaction, high total yield and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
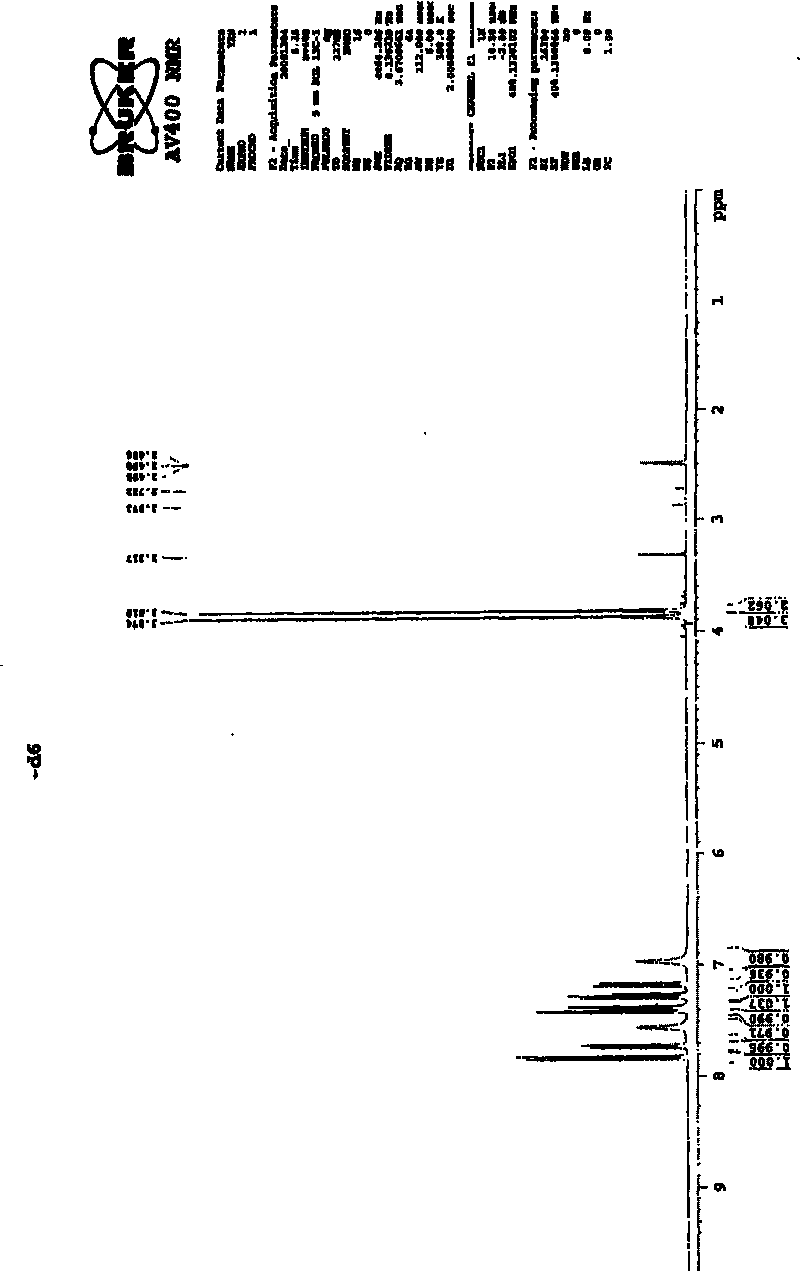
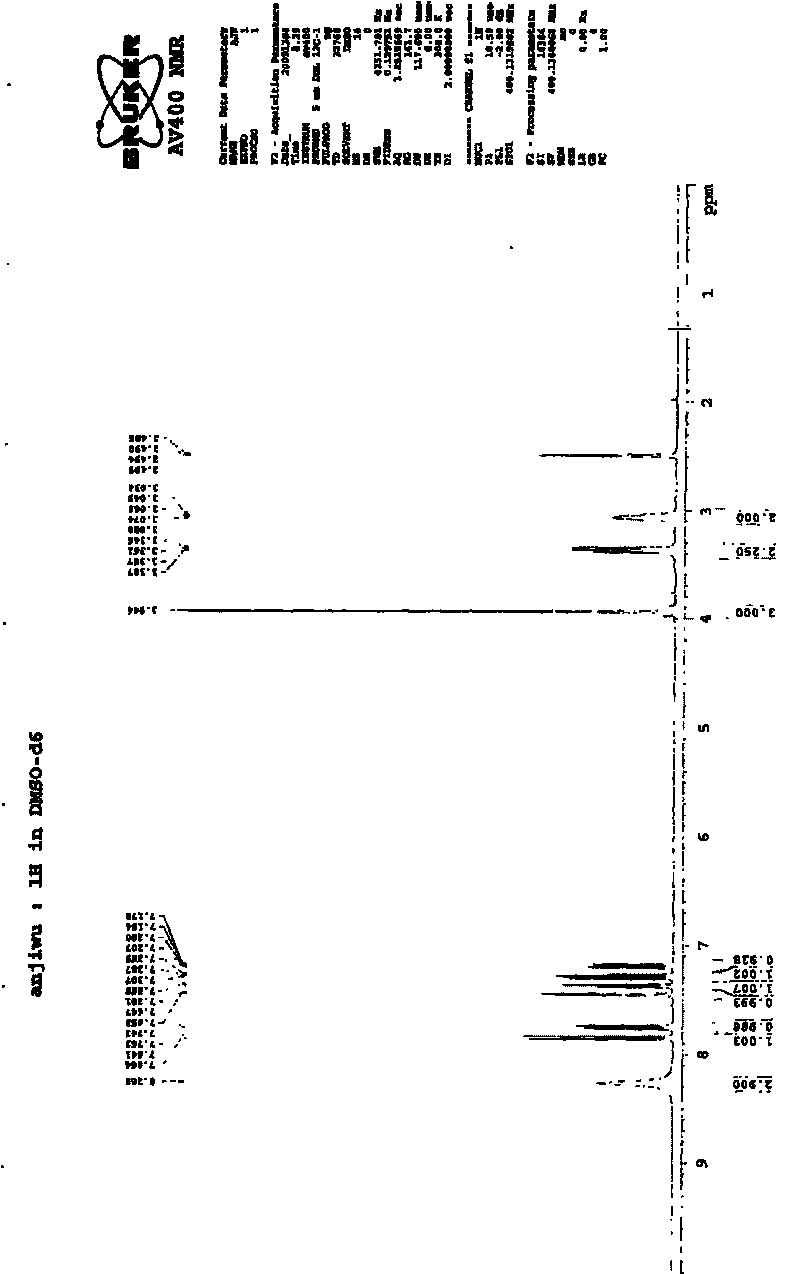
[0024] Add 15g of 2-(7-methoxy-1-naphthyl)acetamide (VII), 5.3g of sodium borohydride, 20.2g of boron trifluoride ethyl ether (47% content), and 300ml of tetrahydrofuran into the reaction flask, and reflux the reaction until the substrate disappears. Pour into dilute hydrochloric acid, extract twice with ethyl acetate, basify the aqueous layer with 10% sodium hydroxide, extract twice with ethyl acetate, combine the oil layers, dry over anhydrous magnesium sulfate, filter, recover ethyl acetate, evaporate Dry to give 2-(7-methoxy-1-naphthyl)ethylamine. Hydrochloric acid-ethyl acetate was added to precipitate a solid, which was filtered to obtain 12 g of off-white solid. The yield was 72.2%. The product is tested with a YRT-3 melting point apparatus, and the melting point of the product is 213-215° C. after testing. Then weigh 10 mg of the resulting product and dissolve it in 0.5 ml of organic solvent (DMSO-d 6 ), using Bruker AV400 to carry out NMR analysis on the sample, t...
Embodiment 2
[0026] Add 15g of 2-(7-methoxy-1-naphthyl)acetamide (VII), 7.6g of potassium borohydride, 101.3g of boron trifluoride ether, and 300ml of tetrahydrofuran into the reaction flask, and reflux until the substrate disappears. Pour into dilute hydrochloric acid, extract twice with ethyl acetate, alkalinize the aqueous layer with 10% sodium hydroxide, extract twice with ethyl acetate, combine the oil layers, dry over anhydrous magnesium sulfate, filter, pass through hydrochloric acid gas, solid Precipitated and filtered to obtain 13 g of off-white solid. The melting point is 214-215°C.
Embodiment 3
[0028] Add 10.8g of 2-(7-methoxy-1-naphthyl)acetamide (VII), 4.8g of sodium borohydride, 22g of aluminum trichloride, and 150ml of tetrahydrofuran into the reaction flask, and reflux until the substrate disappears. Add dilute hydrochloric acid, extract twice with ethyl acetate, alkalinize the aqueous layer with 10% sodium hydroxide, extract twice with ethyl acetate, combine the oil layers, dry over anhydrous magnesium sulfate, filter, add hydrochloric acid-ethyl acetate, solid Precipitated and filtered to obtain 12.8 g of off-white solid. The melting point is 213-215°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com