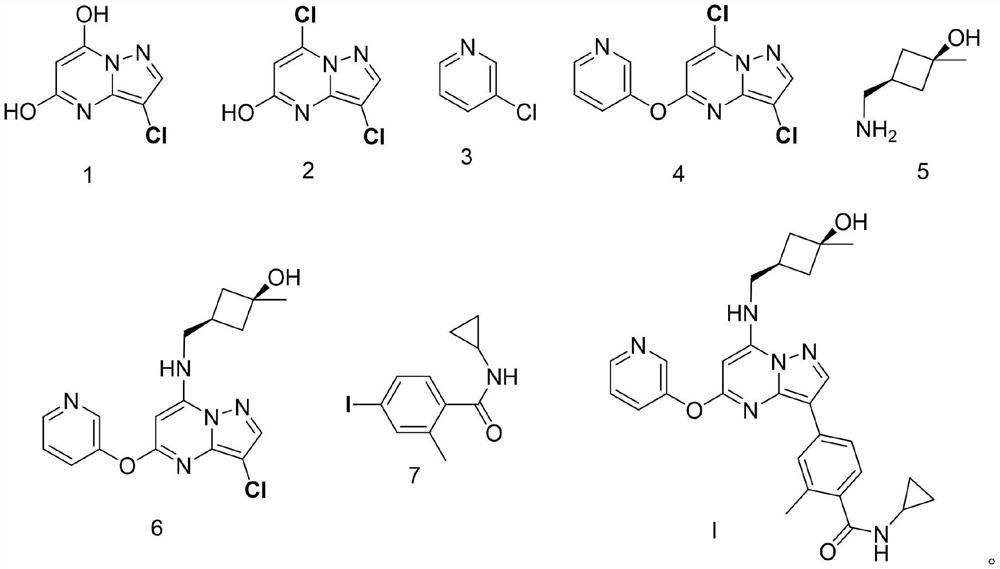
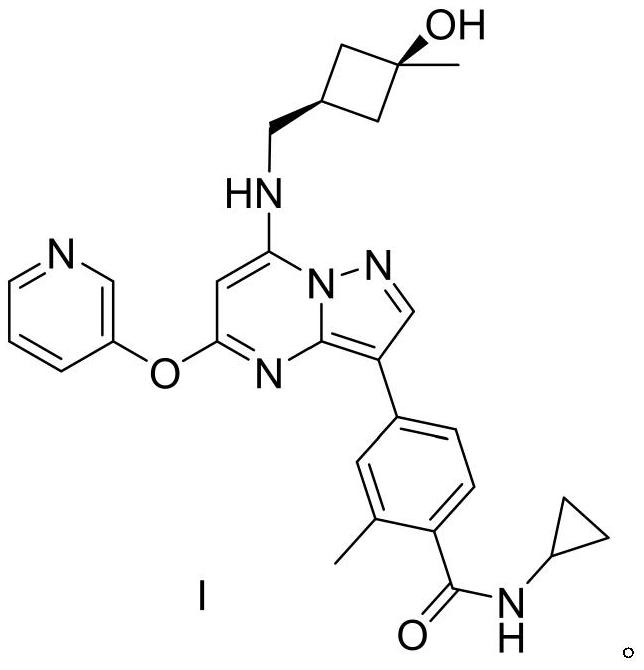
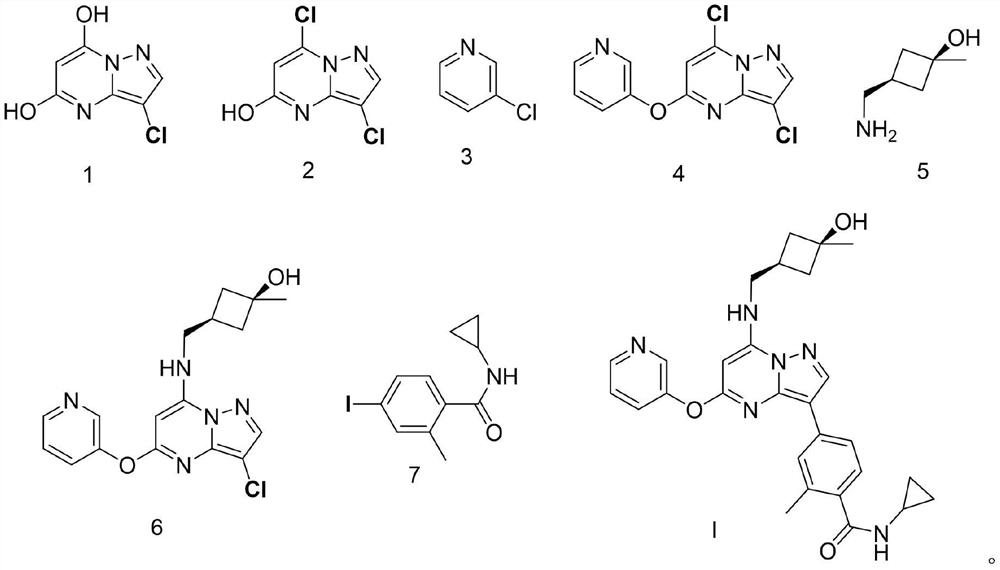
Synthesis and preparation process of antitumor drug CFI-402257
A technology of CFI-402257 and preparation process, which is applied in the field of biomedicine, can solve the problems such as the need to improve the synthesis and preparation process of CFI-402257, and achieve the effects of improving the total yield and industrial operability, reducing the number of reaction steps and improving the selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] Embodiments of the present invention are described in detail below. The embodiments described below are exemplary only for explaining the present invention and should not be construed as limiting the present invention. If no specific technique or condition is indicated in the examples, it shall be carried out according to the technique or condition described in the literature in this field or according to the product specification. The reagents or instruments used were not indicated by the manufacturer, and they were all commercially available conventional products.
[0054] Synthesis of compound shown in embodiment 1 formula 2
[0055] Add the compound shown in formula 1 (5.0g, 26.94mmol) into acetonitrile (30mL) and stir, slowly add POCl 3 (3.1g, 20.21mmol), the mixture was stirred and contacted at room temperature for 12 hours. After the reaction was complete, the mixture was poured on ice, neutralized to a pH value of about 8 with solid sodium bicarbonate, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com