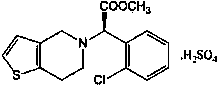
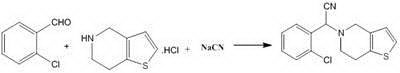Preparation method of clopidogrel intermediate compound
The technology of an intermediate, clopyrrole, is applied in the field of preparation of pharmaceutical intermediates, and can solve the problems of high toxicity of sodium cyanide, unsafe production activities, environmental pollution, etc., achieve low price, maintain dyeing quality, and avoid environmental pollution. effect of the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
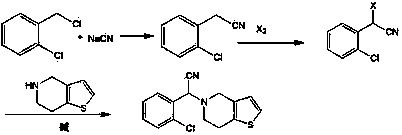
[0022] The synthetic route is as follows:
[0023]
[0024] In a 100ml three-neck flask, add potassium ferrocyanide (1.69g, 4mmol) and p-chlorobenzoyl chloride (3.50g, 20mmol), heat and stir to 180°C for 2 hours, cool to 50°C, and then add o-chlorobenzene dropwise A 30ml mixed solution of formaldehyde (2.81g, 20mmol) and thienopyridine hydrochloride (3.51g, 20mmol) was heated and stirred to 80°C for 8 hours. Suction filtration, the filtrate was concentrated under reduced pressure to remove ethanol, 20ml of water was added, extracted with 20ml*3 dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain (±)-(2-chlorophenyl)- (6,7-Dihydro-4H-thieno[3,2- c ]pyridin-5-yl) acetonitrile crude product, recrystallized from methanol to obtain 4.91 g of off-white product, yield 85%. The resulting product was analyzed by IR and 1 H-NMR identification is as follows: IR spectrum (cm-1): 2227 (-CN); 1 H-NMR (CDCl 3...
Embodiment 2
[0026] The synthetic route is the same as above.
[0027] In a 100ml three-necked flask, add potassium ferrocyanide (1.69g, 4mmol) and benzoyl chloride (2.81g, 20mmol), heat and stir to 160°C for 2 hours, cool to 50°C, and then add o-chlorobenzaldehyde ( 2.81g, 20mmol) and thienopyridine hydrochloride (3.51g, 20mmol) in 30ml of methanol mixed solution, continue heating and stirring to 80 ° C for 8 hours. Suction filtration, the filtrate was concentrated under reduced pressure to remove methanol, 20ml of water was added, extracted with 20ml*3 dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain (±)-(2-chlorophenyl)- (6,7-Dihydro-4H-thieno[3,2- c ] pyridin-5-yl) acetonitrile crude product, recrystallized from methanol to obtain 4.62 g of off-white product, yield 80%.
Embodiment 3
[0029] In a 100ml three-necked flask, add potassium ferrocyanide (1.69g, 4mmol) and benzoyl chloride (2.81g, 20mmol), heat and stir to 160°C for 2 hours, cool to 50°C, and then add o-chlorobenzaldehyde ( 2.81g, 20mmol) and tetrahydrothienopyridine (2.78g, 20mmol) in 30ml of methanol mixed solution, continue heating and stirring to 80 ° C for 8 hours. Suction filtration, the filtrate was concentrated under reduced pressure to remove methanol, 20ml of water was added, extracted with 20ml*3 dichloromethane, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain (±)-(2-chlorophenyl)- (6,7-Dihydro-4H-thieno[3,2- c ]pyridin-5-yl) acetonitrile crude product, recrystallized from methanol to obtain 4.95 g of off-white product, yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com