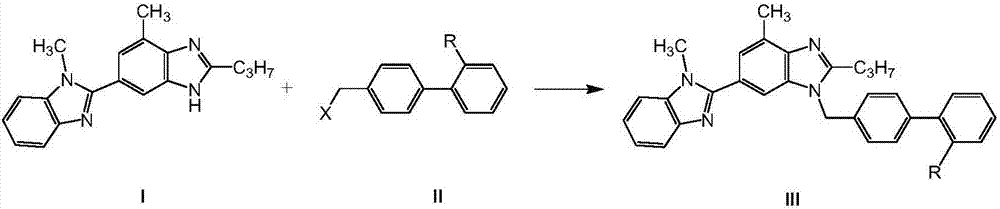
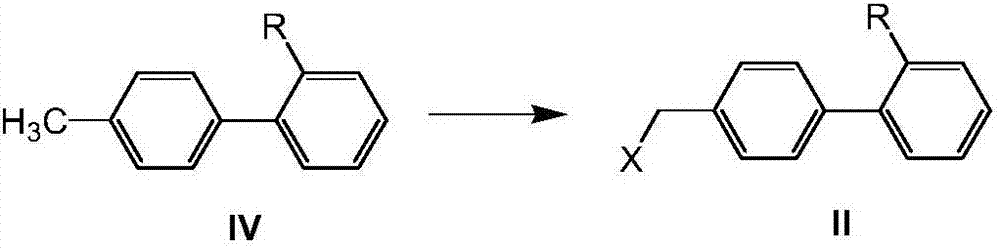
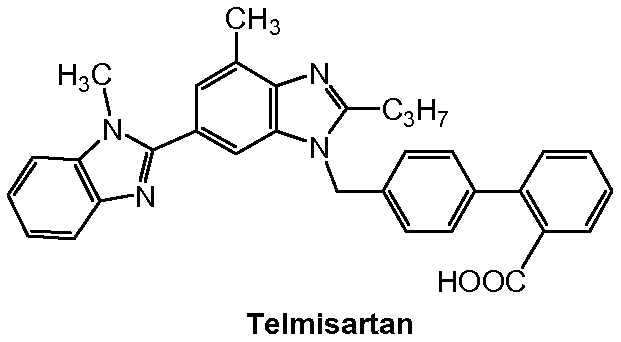
Method for preparing telmisartan and its intermediate
A compound and reaction technology, applied in the field of preparation of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1: Preparation of 4'-chloromethylbiphenyl-2-carboxylic acid (II, R=COOH, X=CI)
[0104] Add 4′-methylbiphenyl 2-carboxylic acid (IV) (10 g, 0.047 mol), azobisisobutylcyanide (AIBN) (0.11 g, 1.5 mol%) into chlorobenzene (35 mL), stir, Heat to 90°C, slowly add SO 2 Cl 2 (3.8mL, 0.047mol) of chlorobenzene solution (15mL), after the dropwise addition, stir for 1 hour, and TLC detects that the reaction is over; the reaction solution is naturally cooled to room temperature, and solids are precipitated, then cooled in an ice bath for 1 hour, and suction filtered , the obtained filtrate was washed with toluene (10 mL×2), and dried to obtain white granular solid II (9.5 g, yield 82%). 1 H NMR (300 MHz, DMSO-d6): 12.79 (s, 1H, OH), 7.21-7.75 (m, 8H, ArH), 4.81 (s, 2H, CH2), MS: 246.1.
Embodiment 2
[0105] Example 2: Preparation of 4'-chloromethylbiphenyl-2-carboxylic acid (II, R=COOH, X=Cl)
[0106] Add 4′-methylbiphenyl 2-carboxylic acid (IV) (10 g, 0.047 mol), azobisisobutylcyanide (AIBN) (0.11 g, 1.5 mol%) into chlorobenzene (70 mL), stir, Heated to 80° C., added trichloroisocyanuric acid (10.9 g, 0.047 mol), stirred and reacted for 12 hours, and insoluble matter was filtered off by heat; the reaction solution was naturally cooled to room temperature, and solids were precipitated, and the resulting filtrate was washed with toluene ( 10mL×2), dried to obtain white granular solid II (6g, yield 52%).
Embodiment 3
[0107] Example 3: Preparation of 4'-chloromethylbiphenyl-2-carboxylic acid (II, R=COOH, X=Cl)
[0108] 4'-methylbiphenyl 2-carboxylic acid (IV) (10 g, 0.047 mol) was dissolved in dichloromethane (150 mL), benzoyl peroxide (0.23 g, 2 mol%) was added, and 1.1 equivalent of chlorine was added, The reaction was stirred for 12 hours; saturated aqueous sodium bicarbonate solution was added to the reaction solution for extraction, the organic layer was separated, and the solvent was evaporated to dryness to obtain off-white solid II (6 g, yield 52%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com