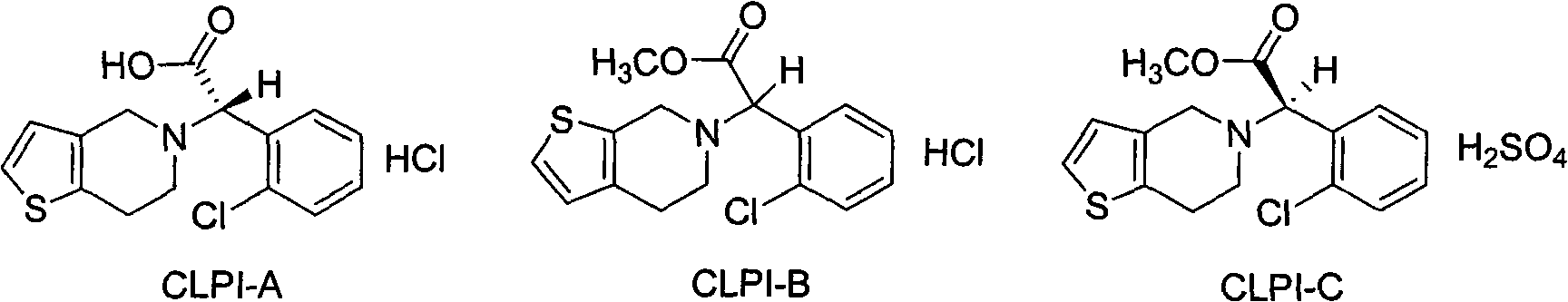
Research and control method of impurity B control method in clopidogrel
A clopidogrel and pyridine technology, applied in the field of research and control of impurity B in clopidogrel, can solve problems such as rare research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
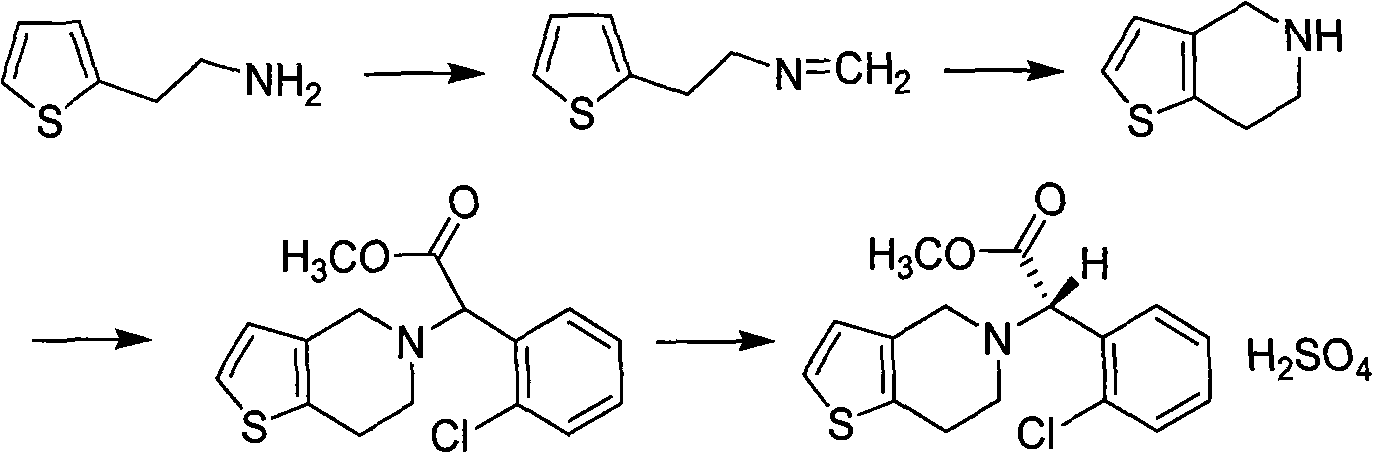
[0026] Embodiment 1, the preparation of 2-thienyl ethyl methyleneamine
[0027] Put 2-thienylethylamine (12.7 g, 0.1 mol) with a 3-thienyl ethylamine content of not more than 0.40% into the reaction flask, and add 36% formaldehyde aqueous solution (10 g, 0.12 mol) dropwise at room temperature under stirring. After dripping, reflux for 3h. Extracted with dichloromethane (150×3), combined extracts, washed with water (50ml), and concentrated to give 2-thienylmethylamine (12.8g, 92%) as light yellow oil.
Embodiment 2
[0028] Example two, preparation of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0029] Put 2-thiopheneethylmethyleneamine (13.9 g, 0.1 mol), 6 mol / L hydrochloric acid (28 ml) into the reaction flask, and stir at room temperature for 6 h. Add sodium hydroxide solution to PH13, extract with dichloromethane (200ml×3), combine the extracts, wash with water (100ml), add anhydrous magnesium sulfate, dry and filter, recover dichloromethane from the filtrate to obtain a light yellow oil crude product (13.9 g, 100%) (literature yield 100%) was directly put into the next step reaction without purification.
Embodiment 3
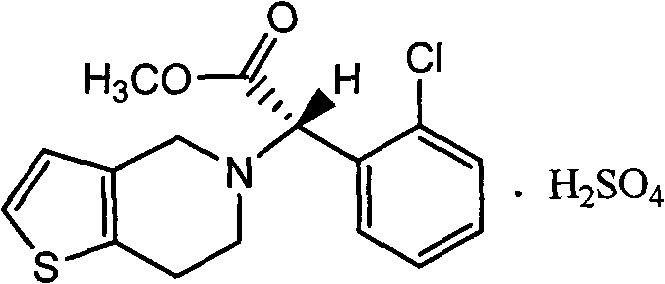
[0030] Example 3, Preparation of 2-(2-chlorophenyl)-2-(4,5,6,7-tetrahydrothiophene[3,2-c]pyridine-5)methyl acetate hydrochloride
[0031] Add 110g of acetonitrile, 11.3g of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine, 20.3g of α-bromo-o-chlorophenylacetic acid methyl ester and 13g of sodium bicarbonate into the reaction flask, and start stirring , and the temperature was raised to 80-85° C. for 5 hours. Cool to 20-25°C, filter, evaporate the filtrate to dryness, add 56g of ethyl acetate, stir, wash with saturated brine 4 times, cool to 5-10°C, add 6.5g of concentrated hydrochloric acid, keep the temperature constant. After the solid was fully separated out, it was suction filtered and dried to obtain methyl 2-(2-chlorophenyl)-2-(4,5,6,7-tetrahydrothiophene[3,2-c]pyridine-5)acetate salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com