Preparation method of salt formation of silodosin intermediate
A compound and high-purity technology, which is applied in the field of silodosin intermediate salt preparation, can solve the problems of poor purification effect, cumbersome steps, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of compound (1) maleate
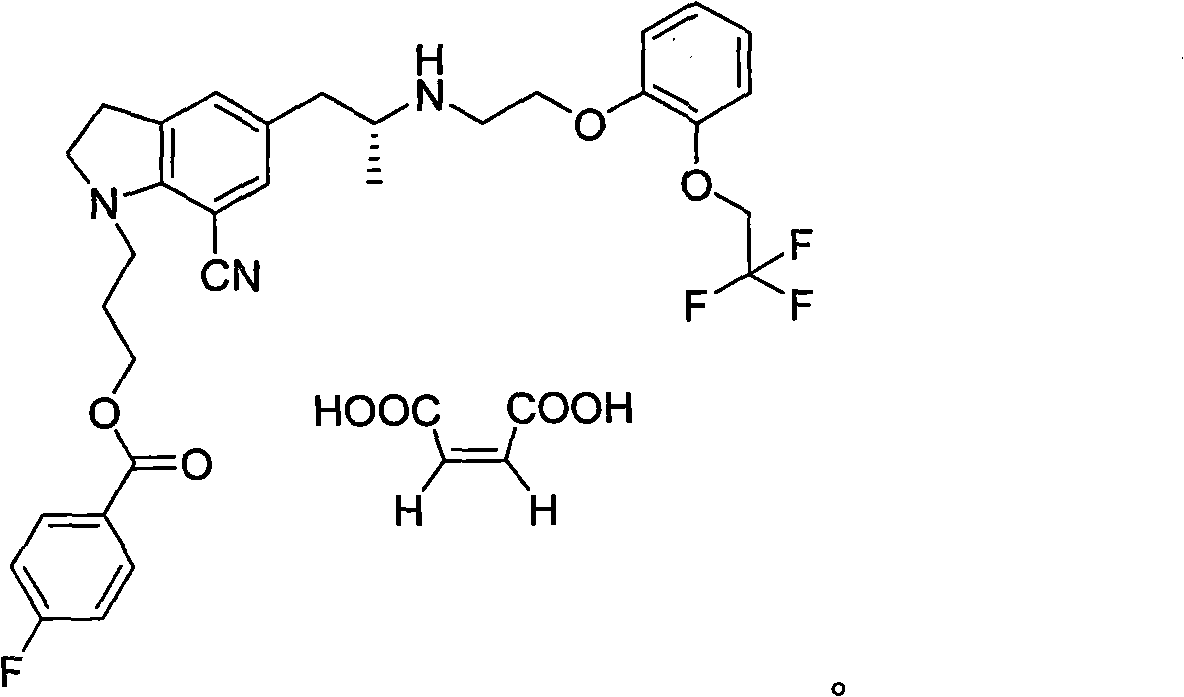
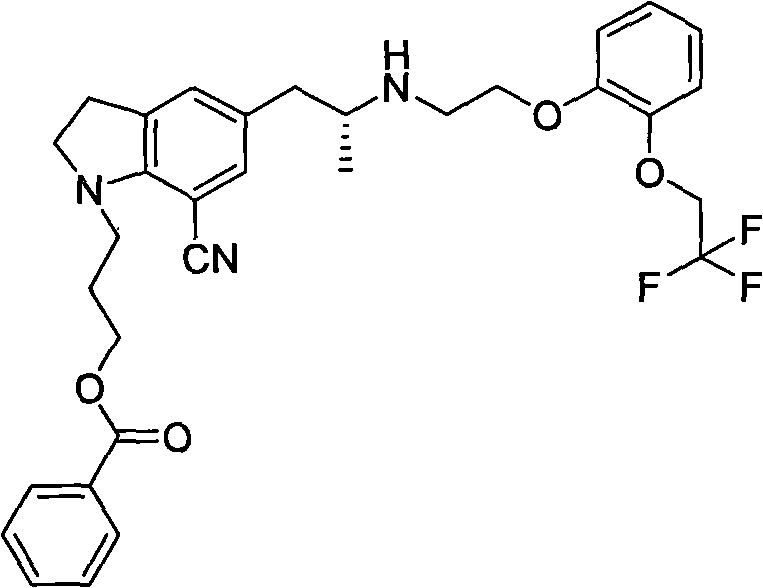
[0026] 10 g of compound (1) and 1.94 g of maleic acid were dissolved in 30 ml of tetrahydrofuran / 60 ml of isopropyl ether, and the mixture was dissolved by heating under reflux. Cool down to 0-15°C and stir, a large amount of solids are precipitated, continue to add 60ml of isopropyl ether. Stir at 5°C for 2h, filter, and dry to obtain solid compound (1) 1-(3-(4-fluorobenzoyl)hydroxypropyl)-5-((2R)-2-(2-(2-( 2,2,2-Trifluoroethoxy)phenoxy)ethylamino)propyl)indoline-7-cyano monomaleate. At this time, the content of the by-product compound (3) in the obtained product was less than 1%, and 10.99 g (yield 92%) was obtained by drying. It can be directly put into the next step reaction.
[0027] The monomaleic acid salt of the compound (1) obtained above was beaten with 100ml isopropyl ether, stirred at 20°C for 3h, filtered and dried to obtain 10.56g of the monomaleic acid salt of the pure compound (1) (yield 88%) )....
Embodiment 2
[0029] Embodiment 2: the preparation of compound (1) maleate
[0030] 10 g of compound (1) and 1.94 g of maleic acid were dissolved in 30 ml of tetrahydrofuran / isopropyl ether, and the mixture was dissolved by heating under reflux. Cool down to 0-5°C and stir, then slowly add 100ml of methyl tert-butyl ether dropwise, a large amount of solids precipitated, continue to add 100ml of methyl tert-butyl ether. Stir at 5°C for 2h, filter, and dry to obtain solid compound (1) 1-(3-(4-fluorobenzoyl)hydroxypropyl)-5-((2R)-2-(2-(2-( 2,2,2-Trifluoroethoxy)phenoxy)ethylamino)propyl)indoline-7-cyano monomaleate. At this time, the content of the by-product compound (3) in the obtained product was less than 0.8%, and 10.38 g (yield 87%) was obtained by drying. It can be directly put into the next step reaction.
[0031] The monomaleic acid salt of the compound (1) obtained above was beaten with 100ml of isopropyl ether, stirred at 20°C for 3h, filtered and dried to obtain 10.03g of the mo...
Embodiment 3
[0032] Embodiment 3: the preparation of silodosin
[0033] Dissolve 8 g of compound (1) monomaleic acid obtained in Example 1 or 2 with 100 ml of DMSO, add 12 ml of 5 mol / L sodium hydroxide, slowly add 7 g of 30% hydrogen peroxide dropwise at 18 to 20° C., and then react at 30° C. , 4h reaction was complete, extracted with ethyl acetate, combined organic layers, extracted with 2N HCl, the obtained aqueous layer was neutralized with sodium hydroxide, extracted with ethyl acetate, washed with saturated sodium bicarbonate, anhydrous sodium sulfate Dry, concentrate under reduced pressure, dissolve in ethyl acetate, cool and crystallize naturally, filter, and dry to obtain 4.9g (88%), with a purity of >99%.
[0034] Mp105~108℃
[0031] The monomaleic acid salt of the compound (1) obtained above was beaten with 100ml of isopropyl ether, stirred at 20°C for 3h, filtered and dried to obtain 10.03g of the monomaleic acid salt of the pure compound (1) (yield 84% ). At this time, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com