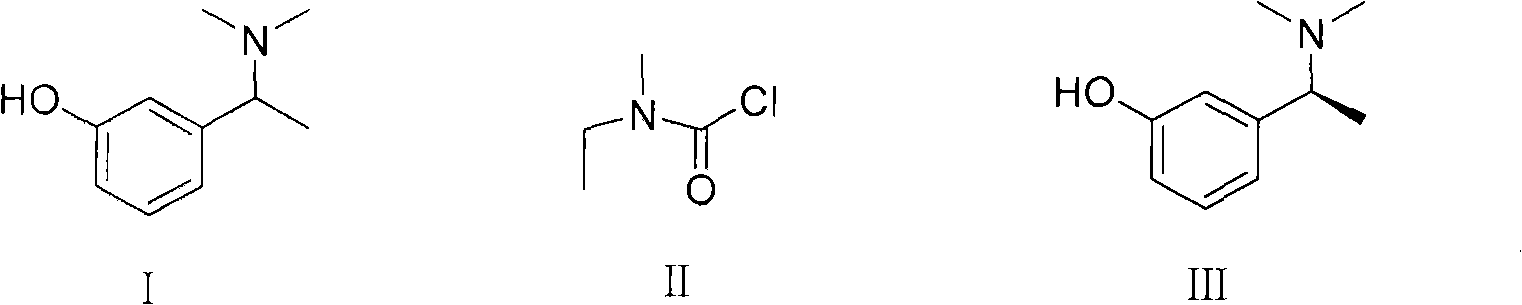
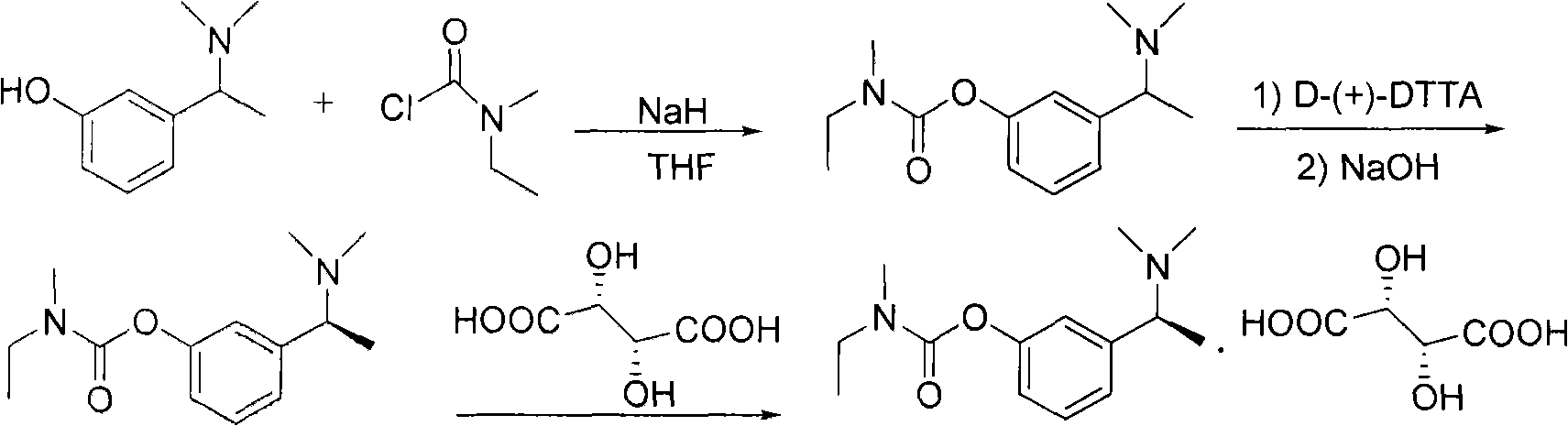
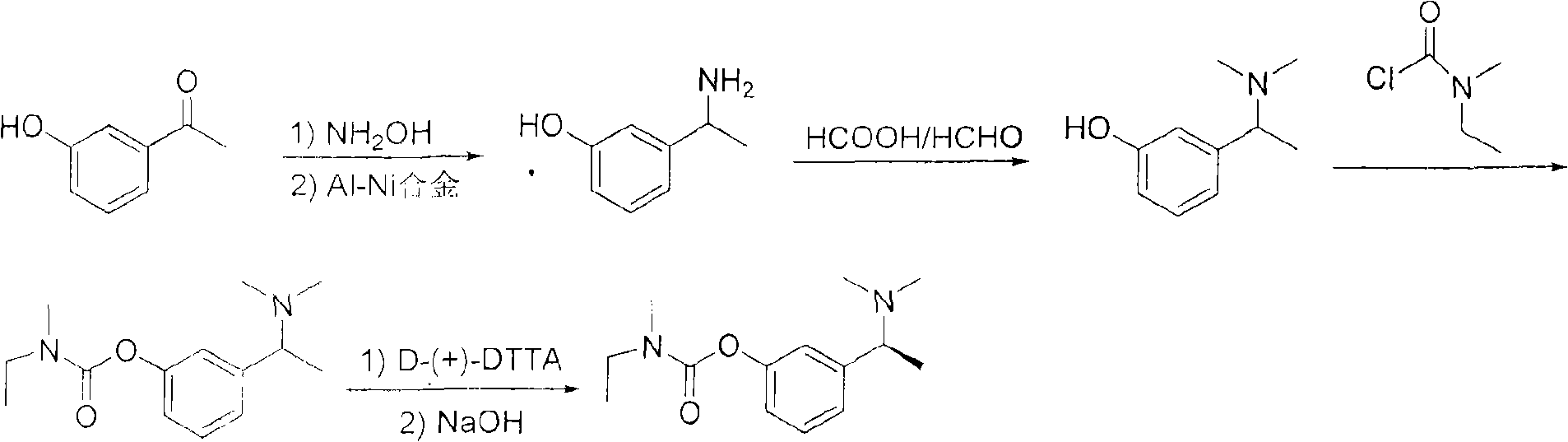
Method for preparing rivastigmine hydrogen tartrate and application thereof
A technology for rivastigmine bitartrate and its effect is applied in the field of pharmaceutical synthesis, can solve the problems of long reduction amide reaction time, limited industrialized production, troublesome post-processing and the like, and achieves the advantages of low cost, simple and easy-to-obtain reagents, and simple and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of 1-(3-methoxyphenyl)ethanol
[0067] Add 100g (0.66mol) of m-methoxyacetophenone into a 1000mL double-necked flask, then add 400mL methanol and 10mL water to dissolve, add NaBH in batches under stirring 4 12.5 g (0.33 mol). After the reaction is complete, distill off methanol, add 150mL of water to shake, adjust the pH to neutral, then extract three times with ethyl acetate or toluene, combine the organic phases, dry over anhydrous sodium sulfate, distill off the solvent to obtain a colorless viscous oil 100g, yield 99%.
Embodiment 2
[0069] Synthesis of 1-(chloroethyl)-3-methoxybenzene
[0070] Add 100 g (0.65 mol) of 1-(3-methoxyphenyl) ethanol into a 500 mL three-necked bottle, slowly add SOCl dropwise 2 60mL (0.82mol), after the dropwise addition, continue to react at room temperature for 1-2h, the reaction is complete, and a yellow turbid liquid is obtained, and the residual SOCl is removed under reduced pressure 2 Add 150mL of water until no bubbles are generated in the bottle, extract three times with 300mL petroleum ether, combine the extracts, wash once with saturated sodium bicarbonate solution and water, dry with anhydrous sodium sulfate, and evaporate the solvent to obtain a yellow transparent oil 107g, yield 95%.
Embodiment 3
[0072] Synthesis of 1-(3-methoxyphenyl)-N,N-dimethylethylamine
[0073] Dissolve 107 g (0.63 mol) of 1-(chloroethyl)-3-methoxybenzene in 900 mL of acetonitrile, add 77 g (0.94 mol) of dimethylamine hydrochloride and 126 g (1.26 mol) of potassium bicarbonate, and heat slowly. Reflux reaction for 12 hours, turn into a yellow solution, filter, distill off the solvent to obtain a red oil, add 200mL of water, stir to lower the pH to 2, extract twice with 400mL ethyl acetate, adjust the pH of the aqueous layer to 10, and then use 500mL ether Extracted three times, combined the organic layers, washed once with water, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 99.4 g of a light red oily substance with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com