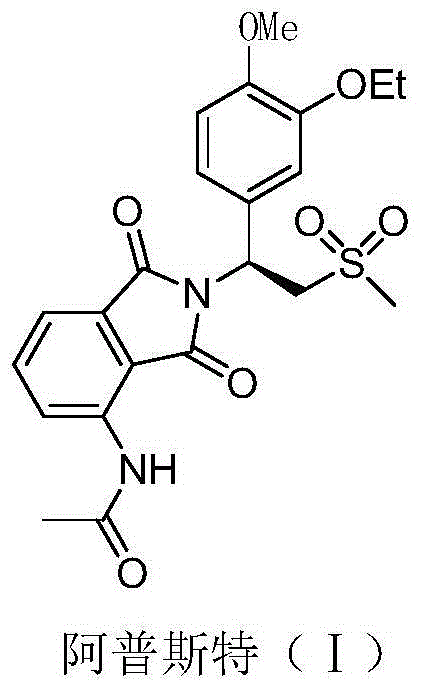
Preparation method for apremilast and intermediate of apremilast
A technology of intermediates and steps, which is applied in the field of preparation of Apremilast and its intermediates, can solve problems such as difficult operation, long reaction process, and reduced molecular utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
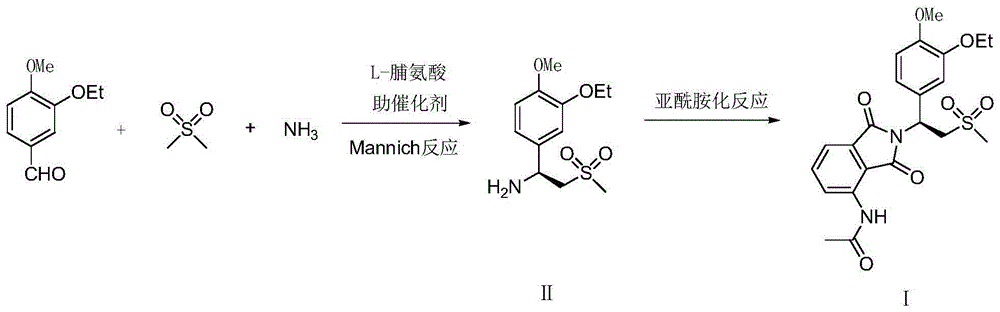
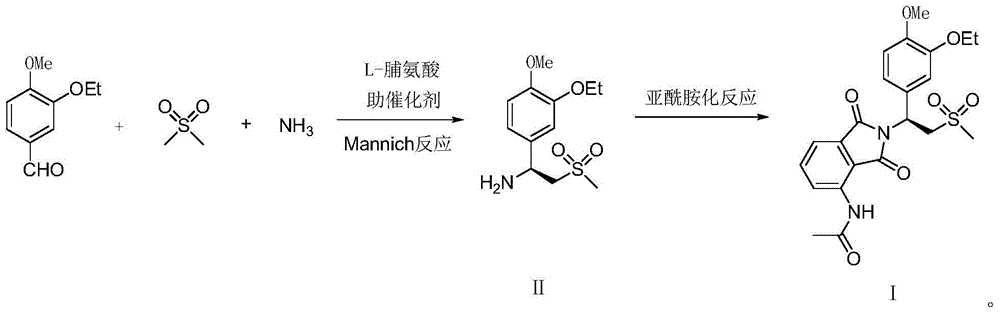
[0049] Example 1: Preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-(methylsulfonyl)ethylamine (II)
[0050] Add 4.5 grams of L-proline, 1.5 grams of D-tartaric acid, and 140 grams of 5% ammonia-tetrahydrofuran solution to a 500-ml flask in turn, stir and dissolve, cool, and add 18.0 grams dropwise to keep the inner temperature between 20-25°C A mixed solution of (0.1 mole) 4-methoxy-3-ethoxybenzaldehyde, 23.5 grams (0.25 moles) dimethyl sulfone and 40 grams of tetrahydrofuran, dripped in about 1 hour, and then reacted at 20-25 ° C for 6 hours . Recover the solvent and ammonia under reduced pressure below 40°C, add 100 grams of water and 200 grams of 1,2-dichloroethane to the residue, stir for 30 minutes, separate the layers, and wash the separated water layer with 1,2-dichloroethane Ethyl chloride was extracted 3 times, 50 grams each time, and the separated aqueous layer could further reclaim L-proline, and the combined organic phase was dried with 20 grams of anhydrous sodi...
Embodiment 2
[0054] Example 2: Preparation of (S)-1-(4-methoxy-3-ethoxy)phenyl-2-(methylsulfonyl)ethylamine (II)
[0055] Add 4.5 grams of L-proline, 1.5 grams of D-tartaric acid, and 140 grams of 5% ammonia-cyclopentyl methyl ether solution to a 500-ml flask in turn, stir and dissolve, cool, and keep the inner temperature between 20-25°C A mixed solution of 18.0 grams (0.1 moles) of 4-methoxyl-3-ethoxybenzaldehyde, 23.5 grams (0.25 moles) of dimethyl sulfone and 40 grams of cyclopentyl methyl ether was added dropwise, and the dripping was completed in about 1 hour. Thereafter, the reaction was incubated at 20-25° C. for 6 hours. Recover the solvent and ammonia under reduced pressure below 40°C, add 100 grams of water and 200 grams of 1,2-dichloroethane to the residue, stir for 30 minutes, separate the layers, and wash the separated water layer with 1,2-dichloroethane Ethyl chloride was extracted 3 times, 50 grams each time, the separated aqueous layer was further reclaimed L-proline, the...
Embodiment 3
[0056] Embodiment 3: the preparation of Apremilast (Ⅰ)
[0057] Add 28.3 grams (0.1 mol, 98.8ee%) (S)-1-(4-methoxyl-3-ethoxyl)phenyl-2-(methylsulfonyl)ethane successively in a dry 250 ml glass flask Amine (II), 22.5 grams of 3-acetamidophthalic anhydride, 120 grams of acetic acid, after stirring for 30 minutes, add 2 grams of perchloric acid, react at 75-80°C for 30 minutes, then raise the temperature and reflux for 2 hours, Evaporate under reduced pressure to recover glacial acetic acid, lower the temperature to 20°C, add 200 grams of saturated saline and 150 grams of ethyl acetate, stir for 30 minutes, separate layers, extract the aqueous layer twice with ethyl acetate, 30 grams each time, combine the organic phase, 10 grams of anhydrous sodium sulfate were dried for 4 hours, filtered, the filtrate was concentrated under reduced pressure, and the residue was recrystallized with 100 grams of isopropanol to obtain 37.5 grams of white solid, apremilast (Ⅰ), yield 79.5%, optical...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com