Visible-light catalytic activity BiOBr-based heterojunction and preparation method thereof
A catalytic activity, heterojunction technology, applied in the direction of chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems that need to be further improved, and achieve the transfer of electrons and the improvement of catalytic activity. Effect of photocatalytic activity and quantum efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Pure BiOBr was prepared as follows:
[0023] ① Mix 0.01 mol of bismuth nitrate with 0.008 mol of 2-bromoethylamine hydrobromide, 0.005 mol of tetramethylammonium bromide and 0.003 mol of urea, and heat to melt to form an ionic liquid;
[0024] ②Heating the ionic liquid described in step ① on an electric furnace until the ionic liquid burns, and the combustion produces a large amount of smoke and emits a large amount of heat;
[0025] ③Collect the solid produced after complete combustion in step ②, cool and grind to obtain a powder, which is designated as sample A.
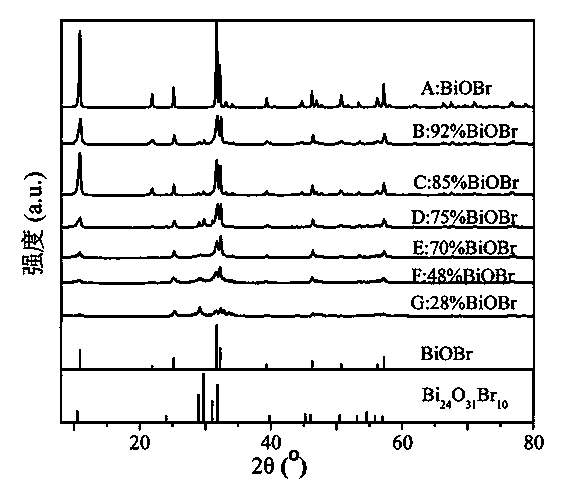
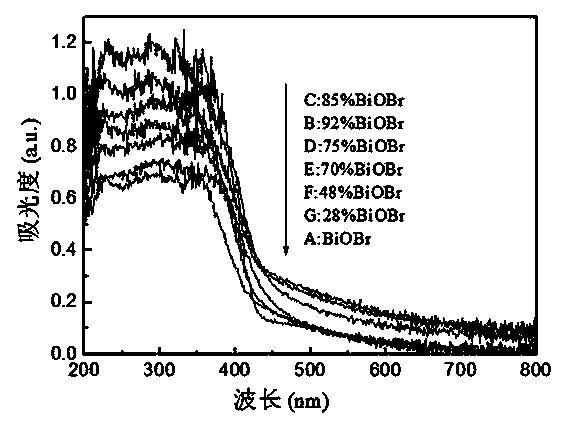
[0026] Sample A was tested by XRD, UV-Vis DRS and SEM respectively, and the test patterns were as follows Figure 1 to Figure 3 shown. figure 1 XRD patterns show that the powder is pure BiOBr. from figure 2 It can be seen that the pure BiOBr prepared in this example has absorption in the visible light region, and the use of α (h ν )=a(h ν -E g ) 2 The formula calculates that its forbidden band w...
Embodiment 2
[0029] Follow the steps below to prepare BiOBr / Bi 24 o 31 Br 10 Heterojunction:
[0030] ① Mix 0.01 mol of bismuth nitrate with 0.0060 mol of 2-bromoethylamine hydrobromide, 0.001 mol of tetraethylammonium bromide and 0.005 mol of urea and heat until melted to form an ionic liquid;
[0031] ②Heating the ionic liquid described in step ① on an electric furnace until the ionic liquid burns, and the combustion produces a large amount of smoke and emits a large amount of heat;
[0032] ③Collect the solid produced after complete combustion in step ②, cool and grind to obtain a powder, which is designated as sample B.
[0033] Carry out XRD and UV-Vis DRS test to sample B, its pattern see respectively figure 1 and figure 2 ,use figure 1 Its peak intensity and BiOBr and Bi 24 o 31 Br 10 The RIR value can be calculated to get BiOBr and Bi 24 o 31 Br 10 The mass fractions were 91.8% and 8.2%, respectively. Depend on figure 2 use α (h ν )=a(h ν -E g ) 2 The formul...
Embodiment 3
[0036] Follow the steps below to prepare BiOBr / Bi 24 o 31 Br 10 Heterojunction:
[0037] ① Mix 0.01 mol of bismuth nitrate with 0.0060 mol of 2-bromoethylamine hydrobromide and 0.008 mol of urea and heat until melted to form an ionic liquid;
[0038] ②Heating the ionic liquid described in step ① on an electric furnace until the ionic liquid burns, and the combustion produces a large amount of smoke and emits a large amount of heat;
[0039] ③Collect the solid produced after complete combustion in step ②, cool and grind to obtain a powder, which is designated as sample C.
[0040] Carry out XRD and UV-Vis DRS test to sample C, its pattern see respectively figure 1 and figure 2 ,use figure 1 Its peak intensity and BiOBr and Bi 24 o 31 Br 10 The RIR value can be calculated to get BiOBr and Bi 24 o 31 Br 10 The mass fractions were 85.2% and 14.8%, respectively. Depend on figure 2 use α (h ν )=a(h ν -E g ) 2 The formula calculates that its forbidden band widt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decolorization rate | aaaaa | aaaaa |
| decolorization rate | aaaaa | aaaaa |
| decolorization rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com